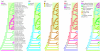Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae) - PubMed (original) (raw)
Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae)
Thomas J Givnish et al. Proc Biol Sci. 2009.
Abstract
The endemic Hawaiian lobeliads are exceptionally species rich and exhibit striking diversity in habitat, growth form, pollination biology and seed dispersal, but their origins and pattern of diversification remain shrouded in mystery. Up to five independent colonizations have been proposed based on morphological differences among extant taxa. We present a molecular phylogeny showing that the Hawaiian lobeliads are the product of one immigration event; that they are the largest plant clade on any single oceanic island or archipelago; that their ancestor arrived roughly 13 Myr ago; and that this ancestor was most likely woody, wind-dispersed, bird-pollinated, and adapted to open habitats at mid-elevations. Invasion of closed tropical forests is associated with evolution of fleshy fruits. Limited dispersal of such fruits in wet-forest understoreys appears to have accelerated speciation and led to a series of parallel adaptive radiations in Cyanea, with most species restricted to single islands. Consistency of Cyanea diversity across all tall islands except Hawai ;i suggests that diversification of Cyanea saturates in less than 1.5 Myr. Lobeliad diversity appears to reflect a hierarchical adaptive radiation in habitat, then elevation and flower-tube length, and provides important insights into the pattern and tempo of diversification in a species-rich clade of tropical plants.
Figures
Figure 1
Representative habit and habitat of the endemic genera/sections of Hawaiian lobeliads: (a) Lobelia gloriamontis (sect. Galeatella), montane bog atop west Maui, (b) Trematolobelia kauaiensis, wet subalpine opening, Kaua `i, (_c_) _Brighamia rockii_, Kapailoa cliffs, Moloka `i, (d) C. hamatiflora (tree with long leaves), streamside cloud forest, east Maui, (e) Cyanea floribunda, cloud-forest understorey, Hawai `i, (_f_) _Clermontia kakeana_, cloud-forest gap, west Maui, and (_g_) _Delissea rhytidosperma_, outplanting from mesic forest, Waimea Canyon, Kaua `i. Photo credits: (a,c) K.R.W., (b,d) K.J.S., (e,f) T.J.G., (g) Vickie Caraway, Hawai `i Department of Land and Natural Resources.
Figure 2
Relationships among Hawaiian lobeliads and allies (_psbA_-trnH, _trnL_-trnF, rpl16, _trnT_-trnL, _trnV_-trnK, _atpB_-rbcL, rbcL partial cds). Phylogram is one of two shortest trees arising from MP analysis of sequence and indel variation, using L. cardinalis and L. vivaldii as out-groups; branch lengths are proportional to the inferred amounts of genetic change. This tree is identical in topology to the parsimony jackknife tree and (except for the position of Isotoma) to the ML and BA trees based on nucleotide characters only (see text); the open arrow indicates the node that collapses in the strict consensus of the two MP trees. Tree length=1393 steps under parsimony; consistency index CI=0.86; CI′=0.76; excluding autapomorphies. Jackknife support is shown above each node; posterior probability is shown below (the single node that collapses in the BA tree has no such probability shown). The Hawaiian lobeliads (green box) are monophyletic with 100% posterior probability.
Figure 3
Evolution of morphological and ecological characters among the Hawaiian lobeliads and close relatives inferred using parsimony, and with L. erinus and Pratia grafted to the bottom of the in-group tree (see the electronic supplementary material). Overlays of inferred ancestral traits illustrate the evolution of (a) fruit type, (b) inflorescence position, (c) habit, (d) pollination syndrome and (e) habitat.
Figure 4
Chronograms illustrating timing of lobeliad evolution based on (a) bottom-up calibration of the in-group molecular phylogeny against asterid fossils and (b) top-down calibration against ages of islands to which individual Hawaiian species are restricted; arrows indicate inferred times and places of initial colonization in the Hawaiian chain. Map shows extant tall islands (green) and submarine contours (blue) associated with original shorelines of islands that are now reduced to pinnacles or reefs in the northwest Hawaiian Islands. Current/former volcanoes are highlighted in red; dated volcanoes are highlighted by triangles. Bars represent ±1 s.d. for age estimates, based on bootstrapping of the in-group data (SD_b_=grey) and variation across out-group trees in calibration set point(s) (SD_c_=magenta). Total s.d.=(SD_b_2+SD_c_2)0.5. SD_c_ falls monotonically towards the present, while SD_b_ first rises and then falls (see the electronic supplementary material).
Figure 5
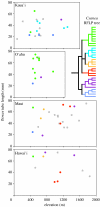
Parallel adaptive radiations in mean elevation and flower-tube length in Cyanea on the four major Hawaiian Islands. Width of the box reflects elevation of island up to the maximum (approx. 2000 m) invaded by most Cyanea species. Dots are colour coded to reflect phylogeny and species membership in the six major clades (inset at right) recognized by Givnish et al. (1995) using plastid restriction site variation; grey dots indicate species (many extinct) not included in that analysis. The six species groups are the Acuminata clade (green); the Hirtella clade (blue); the Solanaceae clade (gold); the Aculeatiflora clade (gold); the Hardyi clade (purple); and the Leptostegia clade (slate blue). The first four clades bear orange fruits and the latter two, purple fruits.
Similar articles
- Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: dynamic photosynthetic responses.
Montgomery RA, Givnish TJ. Montgomery RA, et al. Oecologia. 2008 Mar;155(3):455-67. doi: 10.1007/s00442-007-0936-3. Epub 2008 Jan 22. Oecologia. 2008. PMID: 18210160 - Phylogeny, floral evolution, and inter-island dispersal in Hawaiian Clermontia (Campanulaceae) based on ISSR variation and plastid spacer sequences.
Givnish TJ, Bean GJ, Ames M, Lyon SP, Sytsma KJ. Givnish TJ, et al. PLoS One. 2013 May 2;8(5):e62566. doi: 10.1371/journal.pone.0062566. Print 2013. PLoS One. 2013. PMID: 23658747 Free PMC article. - Patterns of endangerment in the hawaiian flora.
Sakai AK, Wagner WL, Mehrhoff LA. Sakai AK, et al. Syst Biol. 2002 Apr;51(2):276-302. doi: 10.1080/10635150252899770. Syst Biol. 2002. PMID: 12028733 - Molecular biogeography and diversification of the endemic terrestrial fauna of the Hawaiian Islands.
Cowie RH, Holland BS. Cowie RH, et al. Philos Trans R Soc Lond B Biol Sci. 2008 Oct 27;363(1508):3363-76. doi: 10.1098/rstb.2008.0061. Philos Trans R Soc Lond B Biol Sci. 2008. PMID: 18765363 Free PMC article. Review. - Speciation and phylogeography of Hawaiian terrestrial arthropods.
Roderick GK, Gillespie RG. Roderick GK, et al. Mol Ecol. 1998 Apr;7(4):519-31. doi: 10.1046/j.1365-294x.1998.00309.x. Mol Ecol. 1998. PMID: 9628003 Review.
Cited by
- Plastid phylogenomics of Robinsonia (Senecioneae; Asteraceae), endemic to the Juan Fernández Islands: insights into structural organization and molecular evolution.
Cho MS, Yang J, Kim SH, Crawford DJ, Stuessy TF, López-Sepúlveda P, Kim SC. Cho MS, et al. BMC Plant Biol. 2024 Oct 28;24(1):1016. doi: 10.1186/s12870-024-05711-3. BMC Plant Biol. 2024. PMID: 39465373 Free PMC article. - Diversification and trait evolution in New Zealand woody lineages across changing biomes.
Dale EE, Larcombe MJ, Potter BCM, Lee WG. Dale EE, et al. J R Soc N Z. 2022 Aug 17;54(1):98-123. doi: 10.1080/03036758.2022.2108071. eCollection 2024. J R Soc N Z. 2022. PMID: 39439477 Free PMC article. - Islands are key for protecting the world's plant endemism.
Schrader J, Weigelt P, Cai L, Westoby M, Fernández-Palacios JM, Cabezas FJ, Plunkett GM, Ranker TA, Triantis KA, Trigas P, Kubota Y, Kreft H. Schrader J, et al. Nature. 2024 Oct;634(8035):868-874. doi: 10.1038/s41586-024-08036-1. Epub 2024 Oct 16. Nature. 2024. PMID: 39415003 - Adaptive radiation of the Callicarpa genus in the Bonin Islands revealed through double-digest restriction site-associated DNA sequencing analysis.
Setsuko S, Narita S, Tamaki I, Sugai K, Nagano AJ, Ujino-Ihara T, Kato H, Isagi Y. Setsuko S, et al. Ecol Evol. 2024 Sep 13;14(9):e70216. doi: 10.1002/ece3.70216. eCollection 2024 Sep. Ecol Evol. 2024. PMID: 39279792 Free PMC article. - Island biogeography of the megadiverse plant family Asteraceae.
Roeble L, van Benthem KJ, Weigelt P, Kreft H, Knope ML, Mandel JR, Vargas P, Etienne RS, Valente L. Roeble L, et al. Nat Commun. 2024 Aug 23;15(1):7276. doi: 10.1038/s41467-024-51556-7. Nat Commun. 2024. PMID: 39179568 Free PMC article.
References
- Baldwin B.G., Sanderson M.J. Age and rate of diversification of the Hawaiian silversword alliance (Compositae) Proc. Natl Acad. Sci. USA. 1998;95:9402–9406. doi:10.1073/pnas.95.16.9402 - DOI - PMC - PubMed
- Baldwin B.G., Crawford D.J., Francisco-Ortega F., Kim S.-C., Sang T., Stuessy T.F. Molecular phylogenetic insights on the origin and evolution of island plants. In: Soltis D.E., Soltis P.S., Doyle J.J., editors. Molecular systematics of plants II. Kluwer; Boston, MA: 1998. pp. 410–441.
- Brown F.B.H. Origin of the Hawaiian flora. Special Publ. Bernice Pauahi Bishop Mus. 1921;7:131–142.
- Carine M.A., Russell S.J., Santos-Guerra A., Francisco-Ortega J. Relationships of the Macaronesian and Mediterranean floras: molecular evidence for multiple colonizations into Macaronesia and back-colonization of the continent in Convolvulus (Convolvulaceae) Am. J. Bot. 2004;91:1070–1085. doi:10.3732/ajb.91.7.1070 - DOI - PubMed
- Carlquist S. Natural History Press; New York, NY: 1970. Hawaii: a natural history.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources