Recruitment of the RNA helicase RHAU to stress granules via a unique RNA-binding domain - PubMed (original) (raw)
Recruitment of the RNA helicase RHAU to stress granules via a unique RNA-binding domain
Katerina Chalupníková et al. J Biol Chem. 2008.
Abstract
In response to environmental stress, the translation machinery of cells is reprogrammed. The majority of actively translated mRNAs are released from polysomes and driven to specific cytoplasmic foci called stress granules (SGs) where dynamic changes in protein-RNA interaction determine the subsequent fate of mRNAs. Here we show that the DEAH box RNA helicase RHAU is a novel SG-associated protein. Although RHAU protein was originally identified as an AU-rich element-associated protein involved in urokinase-type plasminogen activator mRNA decay, it was not clear whether RHAU could directly interact with RNA. We have demonstrated that RHAU physically interacts with RNA in vitro and in vivo through a newly identified N-terminal RNA-binding domain, which was found to be both essential and sufficient for RHAU localization in SGs. We have also shown that the ATPase activity of RHAU plays a role in the RNA interaction and in the regulation of protein retention in SGs. Thus, our results show that RHAU is the fourth RNA helicase detected in SGs, after rck/p54, DDX3, and eIF4A, and that its association with SGs is dynamic and mediated by an RHAU-specific RNA-binding domain.
Figures
FIGURE 1.
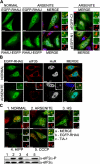
RHAU helicase accumulates in SGs in response to all tested stress stimuli. A, immunofluorescence analysis of RHAU localization in arsenite-induced SGs. HeLa cells, transiently transfected with EGFP-RHAU or RHAU-EGFP for 48 h, were treated with 0.5 m
m
sodium arsenite for 45 min or cultured in normal conditions. After fixation, cells were stained with DAPI (blue) to visualize nuclei and with anti-TIA-1 antibody (red) to detect SGs. Left, distribution of EGFP-RHAU or RHAU-EGFP (green) under normal and arsenite-treated conditions. Panels denoted as MERGE show the digital combination of EGFP, TIA-1, and DAPI images under arsenite-treated conditions; enlargements of_boxed regions_ are shown in small panels indicating merge (a), EGFP (b), and TIA-1 (c). Right, merges of DAPI and TIA-1 with empty vectors pEGFP-C1 or pEGFP-N1;enlargements of boxed regions are shown in small panels as above. B, co-localization of RHAU with eIF3b and HuR in arsenite-induced SGs. HeLa cells were transfected with EGFP-RHAU and treated with or without arsenite as above. The extreme left panels show EGFP-RHAU (green), and the two middle panels are immunofluorescent images for eIF3b (red) and HuR (white;blue in merge), two SG markers. The extreme right panels are their merges. C, effects of different stress stimuli on eIF2α phosphorylation and RHAU localization in SGs. HeLa cells transiently transfected with EGFP-RHAU were cultured without (1) or with the following stress inducers: 0.5 m
m
arsenite for 45 min (2), heat shock (HS) at 42 °C for 45 min (3), 1 μ
m
hippuristanol (HIPP) for 30 min (4), and 1 μ
m
CCCP for 90 min (5). The images show merges of EGFP-RHAU (green) and TIA-1 (red). Enlargements of_boxed regions_ are shown in small panels indicating merge (a), EGFP (b), and TIA-1 (c). The total eIF2α and phosphorylated eIF2α (eIF2_α_-P) were detected by Western blotting. Bar, 10 μm (1 μmin_enlargements_).
FIGURE 2.
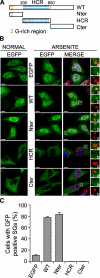
The N-terminal domain of RHAU localizes in SGs. A, scheme of wild-type RHAU and its fragments. Nter, 1-200 aa; HCR, 200-650 aa; Cter, 200-1008 aa. B, intracellular localization of different parts of RHAU. HeLa cells were transfected with an empty pEGFP-C1 vector or plasmids expressing EGFP-fused RHAU (WT) or fragments of RHAU, Nter, HCR, and Cter. After 48 h, cells were cultured in normal conditions or treated with 0.5 m
m
arsenite for 45 min, fixed, and stained for TIA-1 (red). Nuclei were visualized with DAPI (blue). Small panels show enlargements of boxed regions: merge (a), EGFP-fused fragments of RHAU (b) and TIA-1 (c). C, data obtained by quantitative immunofluorescence analysis showing the percentage of transfected cells in which EGFP signals were detected in SGs. Values ± S.E.M. (standard errors of means) were derived from three independent experiments. Bar, 10 μm (1 μmin_enlargements_). GFP, green fluorescent protein.
FIGURE 3.
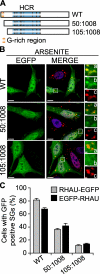
The first N-terminal 105 amino acids of RHAU are necessary for RHAU localization in SG. A, scheme of wild-type RHAU and its deletion mutants. B, intracellular localization of RHAU N-terminal deletion mutants. HeLa cells were transfected with plasmids expressing EGFP-fused RHAU (WT) or N-terminal deletion mutants of RHAU, 50-1008 and 105-1008. After 48 h, cells were treated with 0.5 m
m
arsenite for 45 min, fixed, and stained for TIA-1 (red). Nuclei were visualized with DAPI (blue). Small panels show enlargements of boxed regions: merge (a), EGFP-fused fragments of RHAU (b), and TIA-1 (c). C, quantitative immunofluorescence analysis showing the percentage of transfected cells in which EGFP signals were detected in SGs. Values ± S.E.M. (standard errors of means) were derived from three independent experiments. Bar, 10 μm (1 μmin enlargements a-c). GFP, green fluorescent protein.
FIGURE 4.
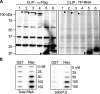
RHAU associates with RNA through its N terminus. A, CLIP method to identify interactions of RHAU with nucleic acids (NA). HeLa cells were transiently transfected with small interfering RNAs against RHAU (siRHAU) or luciferase (siControl). Cells were UV irradiated 72 h later and harvested. Immunoprecipitation was performed with a RHAU antibody. Bound nucleic acids were radiolabeled and detected as a band of ∼120 kDa corresponding to RHAU (lane siControl). However, this band was strongly reduced when endogenous RHAU was depleted (lane siRHAU). Western blot (INPUT) shows efficient RHAU depletion by small interfering RNAs against RHAU. Actin was used as a loading control.B, identification of associated nucleic acids as RNA. After immunoprecipitation, the nucleic acids bound to RHAU were isolated, radiolabeled, and treated with increasing concentrations (0.015, 0.15, 1.5, and 15 units) of RNase A and 1 unit of RQ1DNase. Lane1, isolated and non-treated nucleic acids(NA); lanes 2-5, isolated nucleic acids treated with increasing concentrations of RNase; lane 6, nucleic acids treated with DNase. -Fold changes in intensity ratio (NA/digest NA) are depicted relative to the intensity of non-treated nucleic acids in lane 1, set as 1. Note that nucleic acids are RNase-sensitive but DNase-insensitive. C, RHAU binds to RNA_in vitro_. 0, 2.5, 5, 10, 20, and 40 n
m
GST or GST-RHAU (RHAU) was incubated with 5,000 cpm 5′-end-labeled RNA for 30 min at 37 °C and filtered through nitrocellulose and nylon membranes. The percentage of bound RNA was plotted as a function of increasing concentration of GST-RHAU. Note that dose-dependent RNA binding was seen only with GST-RHAU.D, effect of stress on RHAU association with RNA in vivo. HeLa cells were transfected with EGFP alone or EGFP-WT (a full-length RHAU). Half were treated 24 h later with arsenite (0.5 m
m
for 45 min) followed by UV irradiation and radiolabeling of RHAU-associated nucleic acids. RHAU and associated RNA were analyzed by Western blotting and a phosphorimaging system to detect levels of protein expression and the amount of radiolabeled RNA, respectively. Note that the arsenite treatment (+) did not abolish or dramatically change the RNA binding activity of RHAU.ARS, arsenite. E, comparison of RNA binding activities of wild-type RHAU and its N-terminal deletion mutants. HeLa cells were transfected with EGFP, EGFP-WT, EGFP-(50-1008), or EGFP-(105-1008). Protein and associated RNA were analyzed as in A. Radioactivity of bound RNA was normalized to the expression levels of corresponding proteins. In sharp contrast to constant RNA binding of endogenous RHAU in each lane, N-terminal deletion mutants showed stepwise reduction of RNA interaction compared with WT. ▸, overexpressed EGFP-RHAU or its N-terminal deletion mutants; ▸▸, endogenous RHAU.
FIGURE 5.
Bioinformatics analysis of the RHAU N-terminal domain. A, computational analysis for RNA-binding residues within the RHAU N terminus. The human RHAU amino acid sequence was subjected to three different programs, BindN+, RISP, and RNABindR, available on line. Residues predicted to be positive for RNA binding are indicated by “+” and depicted_above_ the RHAU amino acid sequence. The gray shading corresponds to RNA binding consensus per one amino acid in all three programs. Note that the predicted RNA-binding domain is a long stretch from 3 to 75 aa (gray bar) that overlaps with the G-rich region containing an RGG box motif and with the highly conserved RSM. B, conservation of the RSM through evolution. Orthologs of RHAU used for the multiple sequence alignment were identified as described under “Experimental Procedures.” Similarity of groups was estimated using GeneDoc (version 2.7) with the BLOSUM62 scoring matrix. Red, 90-100% similarity; yellow, 70-90% similarity; and blue, 50-70% similarity. Species and NCBI and Ensembl (in case of X. tropicallis) accession numbers are as follows: human (Homo sapiens, NP_065916), mouse (Mus musculus, NP_082412), chicken (Gallus gallus, XP_422834), frog (Xenopus tropicalis, ENSXETP00000016953), zebrafish (Danio rerio, CAM56669), fruit fly (Drosophila melanogaster, NP_610056), trematoda (Schistosoma japonicum, AAW25528), and choanoflagellate (Monosiga brevicollis, EDQ87802).
FIGURE 6.
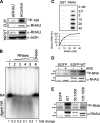
The N-terminal RNA-binding domain is essential and sufficient for RNA interaction in vivo and in vitro. A, CLIP method to show RNA binding activity of the N-terminal 105 amino acid residues of RHAU. HeLa cells were transfected with an empty vector (lane 1), vectors expressing FLAG-tagged RHAU wild type (lane 2), or its fragments, (50-1008) (lane 3), (105-1008) (lane 4), (1-105) (lane 5), and (1-130) (lane 6). Cells were UV irradiated 24 h later, harvested, and immunoprecipitated by FLAG antibody. Bound RNA was labeled with [γ-32P]ATP and detected by a phosphorimaging system as described under “Experimental Procedures.” Left panel, Western blot of immunoprecipitated FLAG-tagged RHAU and its fragments. Right panel, bound RNA. The amount of associated RNA was normalized to the expression level of proteins.▸, FLAG-tagged RHAU and its fragments. B, the N terminus of RHAU binds to RNA in vitro. 0, 25, 50, 100, and 150 n
m
GST or GST-Nter (1-200 aa) was incubated with 10,000 cpm 5′-end-labeled total RNA (left panel) or poly(rU) (right panel) for 30 min at 37 °C and filtered through nitrocellulose and nylon membranes. Note that the dose-dependent RNA binding, both total RNA and poly(rU), was detected only with GST-Nter.
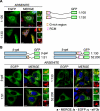
FIGURE 7.
The N-terminal RNA-binding domain is also essential and sufficient for SG localization of RHAU. A, immunofluorescent images of HeLa cells transfected with vectors expressing EGFP-tagged N-terminal fragments of RHAU, (1-105) and (1-130). After 48 h of transfection, cells were treated with arsenite (0.5 m
m
, 45 min) to induce SGs. The merge shows the co-localization of EGFP-tagged RHAU fragments (green) and TIA-1 (red). DAPI (blue) stains nuclei. B, immunofluorescent images of HeLa cells expressing EGFP-β-galactosidase double tagged RHAU N-terminal fragments. Cells were treated as above to induce SGs. The co-localization of EGFP (green) and eIF3b (red) is shown in the merge together with DAPI (blue). Note that only the (1-130) fragment containing an intact RNA-binding domain recruits EGFP-β-galactosidase to SGs. Enlargements of boxed regions are shown in small panels indicating merge (a), EGFP-fused RHAU N-terminal fragment (b), and eIF3b (c).Bar, 10 μm (1 μmin enlargements). GFP, green fluorescent protein.
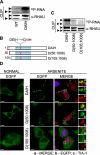
FIGURE 8.
Role of RHAU ATPase activity in RNA binding and retention of protein in SGs. A, RNA binding activity of WT and the ATPase-deficient RHAU mutant DAIH. Levels of RHAU-associated RNA were measured by CLIP as described under “Experimental Procedures” 24 h after transfecting HeLa cells with EGFP-fused WT or DAIH. The level of RNA bound to the DAIH mutant was much higher than that bound to WT. Note that the level of RNA bound to endogenous RHAU is comparable in cells expressing exogenous WT or DAIH. B, schematic representation of N-terminal deletion, DAIH mutants: EGFP-DAIH, EGFP-DAIH(50-1008) (D(50:1008)), and EGFP-DAIH(105-1008) (D(105: 1008)). C, comparison of RNA binding between mutants shown in_B_. HeLa cells were transfected with vectors expressing EGFP-DAIH, EGFP-DAIH(50-1008) (D(50:1008)), or EGFP-DAIH(105-1008) (D(105:1008)), and 24 h later the levels of RNA associated with these mutants and endogenous RHAU were assessed by CLIP as in A. Note that N-terminal deletion mutants show stepwise reduction of RNA binding compared with DAIH full length (such as WT and its deletion mutants in Fig. 4_E_). D, intracellular localization of EGFP-tagged DAIH mutants listed in B in control and arsenite-treated cells. Transfected HeLa cells were treated without (NORMAL) or with 0.5 m
m
sodium arsenite for 45 min. Images denoted MERGE are the merger of EGFP (green), TIA-1 (red), and DAPI (blue). Enlargements of_boxed regions_ show merge (a), EGFP-tagged DAIH mutants (b), and TIA-1 (c). Bar, 10 μm (1 μmin_enlargements_). ▸, EGFP-tagged DAIH and its deletion mutants; ▸▸, endogenous RHAU.
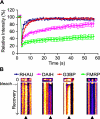
FIGURE 9.
ATP hydrolysis takes part in dynamic RHAU shuttling into and out of SGs. A, fluorescent recovery patterns of RHAU, ATP-deficient RHAU mutant (DAIH), G3BP, and FMRP. HeLa cells transfected with EGFP-tagged RHAU, DAIH, G3BP, or FMRP were treated with arsenite and analyzed by the FRAP method. Bleaching of selected SGs was performed with three scan iterations for a total of 1.5 s. Fluorescence recovery was monitored at 0.5-s intervals for 50 s, and results were analyzed as described under “Experimental Procedures.” Data were collected from at least 10 independent experiments, and each curve represents average fluorescence intensity ±S.E.M. (standard errors of means) over time. B, representative result of FRAP analysis of EGFP-tagged RHAU, DAIH, G3BP, or FMRP. SGs were photobleached, and subsequent fluorescence recoveries are shown in a fire mode in the time scale of recovery. ▴ indicates SGs bleaching.
Similar articles
- Facilitation of mRNA deadenylation and decay by the exosome-bound, DExH protein RHAU.
Tran H, Schilling M, Wirbelauer C, Hess D, Nagamine Y. Tran H, et al. Mol Cell. 2004 Jan 16;13(1):101-11. doi: 10.1016/s1097-2765(03)00481-7. Mol Cell. 2004. PMID: 14731398 - Role of the amino terminal RHAU-specific motif in the recognition and resolution of guanine quadruplex-RNA by the DEAH-box RNA helicase RHAU.
Lattmann S, Giri B, Vaughn JP, Akman SA, Nagamine Y. Lattmann S, et al. Nucleic Acids Res. 2010 Oct;38(18):6219-33. doi: 10.1093/nar/gkq372. Epub 2010 May 14. Nucleic Acids Res. 2010. PMID: 20472641 Free PMC article. - RNA Helicase Associated with AU-rich Element (RHAU/DHX36) Interacts with the 3'-Tail of the Long Non-coding RNA BC200 (BCYRN1).
Booy EP, McRae EK, Howard R, Deo SR, Ariyo EO, Dzananovic E, Meier M, Stetefeld J, McKenna SA. Booy EP, et al. J Biol Chem. 2016 Mar 4;291(10):5355-72. doi: 10.1074/jbc.M115.711499. Epub 2016 Jan 5. J Biol Chem. 2016. PMID: 26740632 Free PMC article. - The conformational plasticity of eukaryotic RNA-dependent ATPases.
Ozgur S, Buchwald G, Falk S, Chakrabarti S, Prabu JR, Conti E. Ozgur S, et al. FEBS J. 2015 Mar;282(5):850-63. doi: 10.1111/febs.13198. Epub 2015 Feb 4. FEBS J. 2015. PMID: 25645110 Review. - Targeting RNA helicases in cancer: The translation trap.
Heerma van Voss MR, van Diest PJ, Raman V. Heerma van Voss MR, et al. Biochim Biophys Acta Rev Cancer. 2017 Dec;1868(2):510-520. doi: 10.1016/j.bbcan.2017.09.006. Epub 2017 Sep 28. Biochim Biophys Acta Rev Cancer. 2017. PMID: 28965870 Free PMC article. Review.
Cited by
- A Helicase Unwinds Hexanucleotide Repeat RNA G-Quadruplexes and Facilitates Repeat-Associated Non-AUG Translation.
Liu H, Lu YN, Paul T, Periz G, Banco MT, Ferré-D'Amaré AR, Rothstein JD, Hayes LR, Myong S, Wang J. Liu H, et al. J Am Chem Soc. 2021 May 19;143(19):7368-7379. doi: 10.1021/jacs.1c00131. Epub 2021 Apr 15. J Am Chem Soc. 2021. PMID: 33855846 Free PMC article. - Principles of Stress Granules Revealed by Imaging Approaches.
Van Treeck B, Parker R. Van Treeck B, et al. Cold Spring Harb Perspect Biol. 2019 Feb 1;11(2):a033068. doi: 10.1101/cshperspect.a033068. Cold Spring Harb Perspect Biol. 2019. PMID: 30709880 Free PMC article. Review. - G-quadruplexes and associated proteins in aging and Alzheimer's disease.
Vijay Kumar MJ, Morales R, Tsvetkov AS. Vijay Kumar MJ, et al. Front Aging. 2023 Jun 1;4:1164057. doi: 10.3389/fragi.2023.1164057. eCollection 2023. Front Aging. 2023. PMID: 37323535 Free PMC article. Review. - Tudor-SN interacts with and co-localizes with G3BP in stress granules under stress conditions.
Gao X, Ge L, Shao J, Su C, Zhao H, Saarikettu J, Yao X, Yao Z, Silvennoinen O, Yang J. Gao X, et al. FEBS Lett. 2010 Aug 20;584(16):3525-32. doi: 10.1016/j.febslet.2010.07.022. Epub 2010 Jul 17. FEBS Lett. 2010. PMID: 20643132 Free PMC article. - BLM helicase protein negatively regulates stress granule formation through unwinding RNA G-quadruplex structures.
Danino YM, Molitor L, Rosenbaum-Cohen T, Kaiser S, Cohen Y, Porat Z, Marmor-Kollet H, Katina C, Savidor A, Rotkopf R, Ben-Isaac E, Golani O, Levin Y, Monchaud D, Hickson ID, Hornstein E. Danino YM, et al. Nucleic Acids Res. 2023 Sep 22;51(17):9369-9384. doi: 10.1093/nar/gkad613. Nucleic Acids Res. 2023. PMID: 37503837 Free PMC article.
References
- Garneau, N. L., Wilusz, J., and Wilusz, C. J. (2007) Nat. Rev. Mol. Cell Biol. 8 113-126 - PubMed
- Anderson, P., and Kedersha, N. (2002) J. Cell Sci. 115 3227-3234 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous