Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease - PubMed (original) (raw)
Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease
Miguel A Hernán et al. Epidemiology. 2008 Nov.
Abstract
Background: The Women's Health Initiative randomized trial found greater coronary heart disease (CHD) risk in women assigned to estrogen/progestin therapy than in those assigned to placebo. Observational studies had previously suggested reduced CHD risk in hormone users.
Methods: Using data from the observational Nurses' Health Study, we emulated the design and intention-to-treat (ITT) analysis of the randomized trial. The observational study was conceptualized as a sequence of "trials," in which eligible women were classified as initiators or noninitiators of estrogen/progestin therapy.
Results: The ITT hazard ratios (HRs) (95% confidence intervals) of CHD for initiators versus noninitiators were 1.42 (0.92-2.20) for the first 2 years, and 0.96 (0.78-1.18) for the entire follow-up. The ITT HRs were 0.84 (0.61-1.14) in women within 10 years of menopause, and 1.12 (0.84-1.48) in the others (P value for interaction = 0.08). These ITT estimates are similar to those from the Women's Health Initiative. Because the ITT approach causes severe treatment misclassification, we also estimated adherence-adjusted effects by inverse probability weighting. The HRs were 1.61 (0.97-2.66) for the first 2 years, and 0.98 (0.66-1.49) for the entire follow-up. The HRs were 0.54 (0.19-1.51) in women within 10 years after menopause, and 1.20 (0.78-1.84) in others (P value for interaction = 0.01). We also present comparisons between these estimates and previously reported Nurses' Health Study estimates.
Conclusions: Our findings suggest that the discrepancies between the Women's Health Initiative and Nurses' Health Study ITT estimates could be largely explained by differences in the distribution of time since menopause and length of follow-up.
Figures
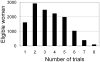
Figure 1
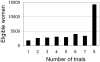
Distribution of eligible women by number of Nurses’ Health Study “trials” of hormone therapy initiation in which they participated
Figure 2
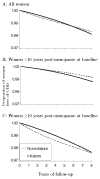
Proportion of women free of CHD by baseline treatment group in the Nurses’ Health Study “trials”
Figure 3
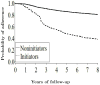
Proportion of women who adhered to their baseline treatment in the Nurses’ Health Study “trials”
Figure 4
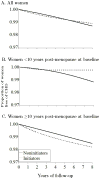
Proportion of women free of CHD under full adherence with the baseline treatment in the Nurses’ Health Study “trials”
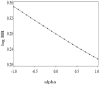
Appendix Figure 1
Sensitivity analysis for lack of adjustment for treatment arm in the inverse probability weighted analysis that adjusts for selection bias due to death between the start of follow-up and the return of questionnaire in the Nurses’ Health Study “trials.” The parameter alpha is the log odds ratio for the hypothesized association between treatment arm and death before returning the questionnaire. Log HR is the log hazard ratio of CHD for initiators versus noninitiators during the first 2 years of follow-up.
Appendix Figure 2
Distribution of eligible women by number of NHS “trials” of hormone therapy discontinuation in which they participated.
Comment in
- Data analysis methods and the reliability of analytic epidemiologic research.
Prentice RL. Prentice RL. Epidemiology. 2008 Nov;19(6):785-8; discussion 789-93. doi: 10.1097/EDE.0b013e318188e83b. Epidemiology. 2008. PMID: 18813015 Free PMC article. - The sound and the fury: was it all worth it?
Hoover RN. Hoover RN. Epidemiology. 2008 Nov;19(6):780-2; discussion 789-93. doi: 10.1097/EDE.0b013e318188e21d. Epidemiology. 2008. PMID: 18813016 - ITT for observational data: worst of both worlds?
Stampfer MJ. Stampfer MJ. Epidemiology. 2008 Nov;19(6):783-4; discussion 789-93. doi: 10.1097/EDE.0b013e318188442e. Epidemiology. 2008. PMID: 18813017
Similar articles
- ITT for observational data: worst of both worlds?
Stampfer MJ. Stampfer MJ. Epidemiology. 2008 Nov;19(6):783-4; discussion 789-93. doi: 10.1097/EDE.0b013e318188442e. Epidemiology. 2008. PMID: 18813017 - Coronary heart disease in postmenopausal recipients of estrogen plus progestin therapy: does the increased risk ever disappear? A randomized trial.
Toh S, Hernández-Díaz S, Logan R, Rossouw JE, Hernán MA. Toh S, et al. Ann Intern Med. 2010 Feb 16;152(4):211-7. doi: 10.7326/0003-4819-152-4-201002160-00005. Ann Intern Med. 2010. PMID: 20157135 Free PMC article. Clinical Trial. - Conjugated equine estrogens and breast cancer risk in the Women's Health Initiative clinical trial and observational study.
Prentice RL, Chlebowski RT, Stefanick ML, Manson JE, Langer RD, Pettinger M, Hendrix SL, Hubbell FA, Kooperberg C, Kuller LH, Lane DS, McTiernan A, O'Sullivan MJ, Rossouw JE, Anderson GL. Prentice RL, et al. Am J Epidemiol. 2008 Jun 15;167(12):1407-15. doi: 10.1093/aje/kwn090. Epub 2008 Apr 29. Am J Epidemiol. 2008. PMID: 18448442 Free PMC article. - The Women's Health Initiative trial and related studies: 10 years later: a clinician's view.
Gurney EP, Nachtigall MJ, Nachtigall LE, Naftolin F. Gurney EP, et al. J Steroid Biochem Mol Biol. 2014 Jul;142:4-11. doi: 10.1016/j.jsbmb.2013.10.009. Epub 2013 Oct 27. J Steroid Biochem Mol Biol. 2014. PMID: 24172877 Review. - Recent epidemiological evidence relevant to the clinical management of the menopause.
Shapiro S. Shapiro S. Climacteric. 2007 Oct;10 Suppl 2:2-15. doi: 10.1080/13697130701606754. Climacteric. 2007. PMID: 17882666 Review.
Cited by
- Review of the target trial methodological approach on treatment effect estimates in kidney failure: protocol for a systematic assessment.
Pinter J, Tunnicliffe DJ, Karunikaikumar P, Anastasiadis A, Hills RK. Pinter J, et al. Syst Rev. 2024 Nov 14;13(1):280. doi: 10.1186/s13643-024-02672-4. Syst Rev. 2024. PMID: 39543770 Free PMC article. - Cost-effectiveness of radiofrequency neurotomy to treat zygapophysial joint pain compared with pain rehabilitation programs.
Hambraeus J, Norström F, Lindholm L. Hambraeus J, et al. Interv Pain Med. 2022 Oct 7;1(4):100147. doi: 10.1016/j.inpm.2022.100147. eCollection 2022 Dec. Interv Pain Med. 2022. PMID: 39238867 Free PMC article. - Hormone Therapy and Biological Aging in Postmenopausal Women.
Liu Y, Li C. Liu Y, et al. JAMA Netw Open. 2024 Aug 1;7(8):e2430839. doi: 10.1001/jamanetworkopen.2024.30839. JAMA Netw Open. 2024. PMID: 39207753 Free PMC article. - Association of Cardiologist Clinic Visits with Cardiovascular Primary Prevention Outcomes Among People with HIV from Underrepresented Racial and Ethnic Groups in the Southern United States.
Durstenfeld MS, Hill CL, Clare RM, Chiswell K, Sanders G, Gray S, Cooper L, Vicini J, Marsolo K, Okeke NL, Meissner EG, Thomas KL, Morse CG, Bloomfield GS, Pettit AC, Longenecker CT. Durstenfeld MS, et al. medRxiv [Preprint]. 2024 Aug 9:2024.08.08.24311709. doi: 10.1101/2024.08.08.24311709. medRxiv. 2024. PMID: 39148821 Free PMC article. Preprint. - Atypical Antipsychotics and the Risk of Sudden Cardiac Death among Individuals Receiving Maintenance Hemodialysis.
Nash RP, Wang L, Gaynes BN, Flythe JE. Nash RP, et al. Gen Hosp Psychiatry. 2023 Nov-Dec;85:148-154. doi: 10.1016/j.genhosppsych.2023.10.016. Epub 2023 Oct 24. Gen Hosp Psychiatry. 2023. PMID: 39108558 Free PMC article.
References
- Ioannidis JP, Haidich AB, Pappa M, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. Jama. 2001;286(7):821–30. - PubMed
- Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–86. - PubMed
- Grodstein F, Stampfer M, Manson J, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease (Erratum in: N Engl J Med 1996;335:1406) New England Journal of Medicine. 1996;335(7):453–61. - PubMed
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Annals of Internal Medicine. 2000;133(12):933–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA087969/CA/NCI NIH HHS/United States
- CA87969/CA/NCI NIH HHS/United States
- R01 HL080644/HL/NHLBI NIH HHS/United States
- R37 AI032475/AI/NIAID NIH HHS/United States
- HL080644/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources