Exploring the nuclear envelope of herpes simplex virus 1-infected cells by high-resolution microscopy - PubMed (original) (raw)
Exploring the nuclear envelope of herpes simplex virus 1-infected cells by high-resolution microscopy
Peter Wild et al. J Virol. 2009 Jan.
Abstract
Herpesviruses are composed of capsid, tegument, and envelope. Capsids assemble in the nucleus and exit the nucleus by budding at the inner nuclear membrane, acquiring tegument and the envelope. This study focuses on the changes of the nuclear envelope during herpes simplex virus 1 (HSV-1) infection in HeLa and Vero cells by employing preparation techniques at ambient and low temperatures for high-resolution scanning and transmission electron microscopy and confocal laser scanning microscopy. Cryo-field emission scanning electron microscopy of freeze-fractured cells showed for the first time budding of capsids at the nuclear envelope at the third dimension with high activity at 10 h and low activity at 15 h of incubation. The mean number of pores was significantly lower, and the mean interpore distance and the mean interpore area were significantly larger than those for mock-infected cells 15 h after inoculation. Forty-five percent of nuclear pores in HSV-1-infected cells were dilated to more than 140 nm. Nuclear material containing capsids protrude through them into the cytoplasm. Examination of in situ preparations after dry fracturing revealed significant enlargements of the nuclear pore diameter and of the nuclear pore central channel in HSV-1-infected cells compared to mock-infected cells. The demonstration of nucleoporins by confocal microscopy also revealed fewer pores but focal enhancement of fluorescence signals in HSV-1-infected cells, whereas Western blots showed no loss of nucleoporins from cells. The data suggest that infection with HSV-1 alters the number, size, and architecture of nuclear pores without a loss of nucleoporins from altered nuclear pore complexes.
Figures
FIG. 1.
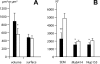
(A) Means and standard deviations of nuclear volume and nuclear surface of HSV-1-infected (▪) and mock-infected (□) cells calculated on the basis of the half axes measured on confocal images. (B) The number of nuclear pores counted on cryo-FESEM images (NP-SEM) were calculated per nuclear surface of confocal images, and the mean number of fluorescent signals was determined on nuclei labeled with Mab414 (NP-414) or with antibodies against Nup153 (NP-153) as described in Materials and Methods. Means are statistically different (*, P < 0.05; **, P < 0.001; number of nuclei = 10) compared to mock-infected cells.
FIG. 2.
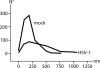
Frequency distribution of the interpore distance in 10 HSV-1- and 10 mock-infected cells measured on cryo-FESEM images (60 distances on average). The mean interpore distance is 364 nm in HSV-1-infected cells and 208 nm in mock-infected cells.
FIG. 3.
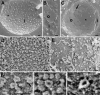
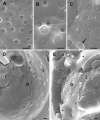
Images of nuclear surfaces of mock- and HSV-1-infected Vero cells (A to C) and HeLa cells (D to H) 15 h postinfection obtained by cryo-FESEM after freeze fracturing (A to C) or by FESEM after dry fracturing (D to H) showing remarkable reductions in the number of nuclear pores/μm2 and enlargement of the maximal interpore area (asterisks) in HSV-1-infected cells (C and E) compared to mock-infected cells (A and D). Nuclear pores appear on the inner nuclear membrane (i) as round buttons but as a distinct low depression at the outer nuclear membrane (o) in mock-infected cells (A and B). In HSV-1-infected cells, the NPC and/or nuclear material protrudes through normal-sized and enlarged nuclear pores, and a distinctly bordered hole (arrow) was formed as apparent at the outer nuclear surface (C). The overall diameter of the NPC is enlarged and the central pore channel is dilated in dry-fractured HSV-1-infected cells (G and H) compared to NPCs of mock-infected cells (F). Bars, 200 nm.
FIG. 4.
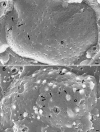
Cryo-FESEM images showing the inner (i) and parts of the outer (o) nuclear membranes of HSV-1-infected HeLa cells at 10 h of incubation. (A) Nuclear pores appear as button-like structures or holes. There are also slightly larger holes with distinct borders and small protrusions of similar size (arrows) as well as three holes with diameters of up to 250 nm and a few budding capsids. (B) The number of budding capsids is about 1.8/μm2. One budding is probably close to completion (arrowhead). There are holes from 110 to 220 nm, two protrusions (arrows) of 130 nm in diameter, and folds of the nuclear surface. Bars, 200 nm.
FIG. 5.
Cryo-FESEM images of the outer nuclear membrane (o) of mock-infected (A) and HSV-1-infected (B) HeLa cells after 10 h of incubation. (A) Nuclear pores appear as round, small depressions with a distinct NPC structure, and a few appear as small buttons (arrows). (B) Pores appear either as small but deep depressions without obvious NPC structure or as buttons. Some of them have larger diameters and are over top the nuclear membrane (arrows). In addition, there are holes with distinct borders (arrowheads) and numerous budding capsids. The outer nuclear membrane is locally broken away, giving view to the inner nuclear membrane (i). Bars, 200 nm.
FIG. 6.
Details of the outer nuclear membrane shown in Fig. 3 (A to C) and cryo-FESEM images of HSV-1-infected Vero cells after 10 h (D) and 17 h (E) of incubation. (A) Nuclear pores of mock-infected cells with NPC structures. (B and C) Nuclear pores either miss NPC structures or are completely filled with NPCs and/or nuclear material that protrudes into the cytoplasm of HSV-1-infected cells. The hole (arrow) does not give a view onto the inner nuclear membrane. (D) Large areas are devoid of nuclear pores, the number of which is low, and appear as buttons at both the inner (i) and outer (o) nuclear membranes. (E) Large areas (asterisks) devoid of pores bulge into the cytoplasm. Bars, 200 nm.
FIG. 7.
Cryo-FESEM and CFTEM images of HSV-1-infected Vero cells at 15 h (A) and 17 h (B to E) of infection. (A) Inner nuclear membrane (i) with a single budding capsid, normal-sized nuclear pores with material protruding through some of them, and indentations (arrows) with a smooth transition to the inner nuclear membrane indicating that these resulted from budding. (B) Cross-fracture plane through a nucleus showing a gap of 560 nm bound by the inner (i) and outer (o) nuclear membranes. (C to E) CFTEM images of a normal nuclear pore with clearly visible pore complex (C), a gap with boundaries formed by the inner and outer nuclear membranes (D), and nuclear material (arrow) together with capsids protruding through a gap into the cytoplasm close to an RER cisterna. Bars, 200 nm.
FIG. 8.
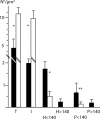
Means and standard deviations of intact and impaired nuclear pore numbers expressed per μm2 nuclear surface in HSV-1-infected (▪) and mock-infected (□) cells incubated for 15 h analyzed on cryo-FESEM images. T, total pores; I, intact pores; H < 140 and P < 140, holes and pores <140 nm in diameter, respectively; H > 140 and P > 140, holes and pores >140 nm in diameter, respectively. Data for each morphological entity were compared by the Student t test (*, P < 0.0001; **, P < 0.05; n = 10).
FIG. 9.
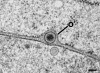
CFTEM micrograph of a Vero cell at 12 h of infection showing an enveloped virion within the perinuclear space forcing the outer nuclear membrane (o) into the cytoplasm. Bar, 100 nm.
FIG. 10.
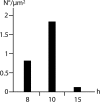
Number of budding capsids per μm2 at the nuclear envelope at 8, 10, and 15 h of incubation.
FIG. 11.
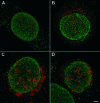
Confocal microscopic images of mock-infected cell at 15 h (A) and of HSV-1-infected HeLa cells recognized by staining of the tegument protein VP16 (red) at 8 h (B) or 15 h (C and D) postinoculation immunostained for nucleoporins (green) with Mab414 (A, B, and C) or Nup153 (D). Nucleoporin distribution is regular in mock-infected cells when stained with Mab414 (A) or with Nup153 (not shown). In HSV-1-infected cells, nucleoporin distribution is irregular, the fluorescence signal is focally enhanced, and the number of signals is reduced, as apparent at 15 h of incubation (C and D). Bar, 1 μm.
FIG. 12.
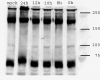
Western blots of mock- and HSV-1-infected HeLa cells 0 to 24 h postinoculation as described in Materials and Methods using Mab414 showing no apparent differences between mock- and HSV-1-infected cells at any time of infection.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Identification of a novel neurovirulence factor encoded by the cryptic orphan gene UL31.6 of herpes simplex virus 1.
Kato A, Iwasaki R, Takeshima K, Maruzuru Y, Koyanagi N, Natsume T, Kusano H, Adachi S, Kawano S, Kawaguchi Y. Kato A, et al. J Virol. 2024 Jul 23;98(7):e0074724. doi: 10.1128/jvi.00747-24. Epub 2024 May 31. J Virol. 2024. PMID: 38819171 Free PMC article. - Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.
Pillay J, Gaudet LA, Saba S, Vandermeer B, Ashiq AR, Wingert A, Hartling L. Pillay J, et al. Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article. - The effect of tobacco ingredients on smoke chemistry. Part I: Flavourings and additives.
Baker RR, Pereira da Silva JR, Smith G. Baker RR, et al. Food Chem Toxicol. 2004;42 Suppl:S3-37. doi: 10.1016/S0278-6915(03)00189-3. Food Chem Toxicol. 2004. PMID: 15072836 - Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.
Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Triana L, et al. Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
- Nuclear Egress.
Draganova EB, Thorsen MK, Heldwein EE. Draganova EB, et al. Curr Issues Mol Biol. 2021;41:125-170. doi: 10.21775/cimb.041.125. Epub 2020 Aug 7. Curr Issues Mol Biol. 2021. PMID: 32764158 Free PMC article. Review. - Epstein-Barr virus protein kinase BGLF4 targets the nucleus through interaction with nucleoporins.
Chang CW, Lee CP, Huang YH, Yang PW, Wang JT, Chen MR. Chang CW, et al. J Virol. 2012 Aug;86(15):8072-85. doi: 10.1128/JVI.01058-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623767 Free PMC article. - Functional Identification and Characterization of the Nuclear Egress Complex of a Gammaherpesvirus.
Lv Y, Shen S, Xiang L, Jia X, Hou Y, Wang D, Deng H. Lv Y, et al. J Virol. 2019 Nov 26;93(24):e01422-19. doi: 10.1128/JVI.01422-19. Print 2019 Dec 15. J Virol. 2019. PMID: 31554685 Free PMC article. - Environmental Stability and Transmissibility of Enveloped Viruses at Varied Animate and Inanimate Interfaces.
Zeng L, Li J, Lv M, Li Z, Yao L, Gao J, Wu Q, Wang Z, Yang X, Tang G, Qu G, Jiang G. Zeng L, et al. Environ Health (Wash). 2023 May 30;1(1):15-31. doi: 10.1021/envhealth.3c00005. eCollection 2023 Jul 21. Environ Health (Wash). 2023. PMID: 37552709 Free PMC article. Review. - Role of Vesicle-Associated Membrane Protein-Associated Proteins (VAP) A and VAPB in Nuclear Egress of the Alphaherpesvirus Pseudorabies Virus.
Dorsch AD, Hölper JE, Franzke K, Zaeck LM, Mettenleiter TC, Klupp BG. Dorsch AD, et al. Viruses. 2021 Jun 10;13(6):1117. doi: 10.3390/v13061117. Viruses. 2021. PMID: 34200728 Free PMC article.
References
- Allen, T. D., G. R. Bennion, S. A. Rutherpord, S. Reipert, A. Ramalho, E. Kiseleva, and M. W. Goldberg. 1997. Macromolecular substructure in nuclear pore complexes by in-lens field-emission scanning electron microscopy. Scanning 19403-410. - PubMed
- Allen, T. D., and M. W. Goldberg. 1993. High-resolution SEM in cell biology. Trends Cell Biol. 3205-208. - PubMed
- Allen, T. D., S. A. Rutherford, G. R. Bennion, C. Wiese, S. Riepert, E. Kiseleva, and M. W. Goldberg. 1998. Three-dimensional surface structure analysis of the nucleus. Methods Cell Biol. 53125-138. - PubMed
- Ball, J. R., and K. S. Ullman. 2005. Versatility at the nuclear pore complex: lessons learned from the nucleoporin Nup153. Chromosoma 114319-330. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources