Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection - PubMed (original) (raw)
Review
. 2008 Sep;60(3):358-403.
doi: 10.1124/pr.107.00107.
Frank P Bymaster, Herbert Y Meltzer, Ariel Y Deutch, Gary E Duncan, Christine E Marx, June R Aprille, Donard S Dwyer, Xin-Min Li, Sahebarao P Mahadik, Ronald S Duman, Joseph H Porter, Josephine S Modica-Napolitano, Samuel S Newton, John G Csernansky
Affiliations
- PMID: 18922967
- PMCID: PMC4821196
- DOI: 10.1124/pr.107.00107
Review
Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection
Jeffrey A Lieberman et al. Pharmacol Rev. 2008 Sep.
Erratum in
- Pharmacol Rev. 2008 Dec;60(4):582
Abstract
Various lines of evidence indicate the presence of progressive pathophysiological processes occurring within the brains of patients with schizophrenia. By modulating chemical neurotransmission, antipsychotic drugs may influence a variety of functions regulating neuronal resilience and viability and have the potential for neuroprotection. This article reviews the current literature describing preclinical and clinical studies that evaluate the efficacy of antipsychotic drugs, their mechanism of action and the potential of first- and second-generation antipsychotic drugs to exert effects on cellular processes that may be neuroprotective in schizophrenia. The evidence to date suggests that although all antipsychotic drugs have the ability to reduce psychotic symptoms via D(2) receptor antagonism, some antipsychotics may differ in other pharmacological properties and their capacities to mitigate and possibly reverse cellular processes that may underlie the pathophysiology of schizophrenia.
Figures
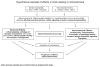
Fig. 1
Overview of the possible mechanisms of neurodegeneration occurring in schizophrenia. Italic denotes potential areas of intervention by antipsychotic drugs.
Fig. 2
A summary of the intracellular signaling cascades that occur within neurons and glia within the brain. Schizophrenia has been associated with dysregulation at a number of loci along these signaling pathways, and antipsychotic drugs may act to reverse some of the pathological changes that have been observed. This slide provides a summary of signaling cascades that occur within neurons and glial cells in the brain that may contribute to schizophrenia, although not all of the cascades shown will be found in a given cell or pathway. The left side summarizes the excitatory and inhibitory iontropic receptors. The top illustrates components of the two key signaling cascades associated with G-protein-coupled metabotropic receptors including adenylyl cyclase and phospholipase C activation. The bottom demonstrates the apoptosis cascade and specific neurotrophic factor-receptor interactions. The right side summarizes some of the key molecules involved in oxidative stress. AP-1, activator protein-1 complex; BZ, benzodiazepines; CAT, catalase; Cl−, chloride; CR, calretinin; Cyt c, cytochrome c; DAG, diacylglycerol; ER, endoplasmic reticulum; GRK, G-protein-coupled receptor kinases; GSH-Px, glutathione peroxidase; H2O2, hydrogen peroxide; IPSP, inhibitory postsynaptic potential; KYN, kynurenic acid; M, muscarinic acetylcholine receptors; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; nAChR, nicotinic acetylcholine receptor; NE, norepinephrine; NS, neuroactive steroids; O2-, superoxide radical; -OH, hydroxyl anion; P, phosphorylation; PI3K, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol triphosphate; PP2A, protein phosphatase 2A; T, transporter protein; Vit, vitamin.
Fig. 3
Differential effects of 90-day treatment with FGAs [haloperidol (HAL)] and SGAs [risperidone (RISP) and clozapine (CLOZ)] on cerebral cortical NGF and ChAT. Animals received each drug (HAL = 2 mg/kg/day; RISP = 2.5 mg/kg/day; CLOZ = 20 mg/kg/day) in drinking water continuously for 90 days. Some animals treated with HAL for 45 days were switched to either RISP (HAL/RISP) or CLOZ (HAL/CLOZ) administration, and some animals with RISP and CLOZ administration for 45 days were switched to HAL treatment (RISP/HAL and CLOZ/HAL, respectively) for the next 45 days to investigate the restoration or prevention, respectively, of HAL-induced reduction of NGF and ChAT. Plasma levels of drugs were similar to plasma drug levels reported in patients with schizophrenia at therapeutic doses, and all the immunohistochemical procedures were done as described previously (Parikh et al., 2004a). Immunohistograms show NGF (red) in cortical neuronal cell bodies that are surrounded by cholinergic projections (ChAT, green) of cholinergic neurons from nucleus basalis. CO (vehicle-treated) shows dense localization of NGF and ChAT. HAL shows very significant reductions in both NGF and ChAT. However, RISP shows a slight reduction, whereas no reduction was found with CLOZ. Furthermore, post-treatment with RISP or CLOZ shows significant restoration (HAL/RISP < HAL/CLOZ) of HAL-induced reduction of NGF and ChAT. Likewise, pretreatment with RISP or CLOZ shows significant prevention (RISP/HAL < CLOZ/HAL) of HAL-induced reduction of NGF and ChAT. The detailed quantitative data were reported earlier (Parikh et al., 2004a,b).
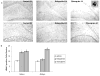
Fig. 4
Differential temporal effects of haloperidol (HAL) and olanzapine (OLZ) administration on cell proliferation in hippocampus of adult rat brain. Animals were treated with vehicle, HAL or OLZ, as described in Fig. 4 for 14 and 45 days. All procedures were as described in Wakade et al. (2002). Newly born cells were labeled with bromodeoxyuridine (BrdU) and visualized with DAB immunostaining staining (brown dots), and then stained cells were counted. A, top shows the representative immunohistograms of control-14 day, HAL-14 day, and olanzapine-14 day; bottom shows the control-45 day, HAL-45 day, and olanzapine-45 day. Most of the proliferating cells are in the hilus and subgranular zone of the dentate gyrus. The inset in olanzapine-45 day shows a higher magnification of a group of BrdU-positive cells. B, differential temporal effects on the numbers of proliferating cells (*, p < 0.001 versus vehicle).
Fig. 5
Generalization of comparative changes of FGAs and SGAs on physiological processes thought to be dysfunctional in schizophrenia. These comparative observations are based on either changes found in patients with schizophrenia or inferred from in vitro or animal studies cited in this review. In some instances, the effects of the drugs within the class differ, and this is denoted by the use of two symbols. NC, no change;+, increase or improvement; −, decrease or decline.
Similar articles
- Real-World Treatment of Schizophrenia in Adults With a 22q11.2 Microdeletion: Traitement dans le monde réel de la schizophrénie chez des adultes atteints du syndrome de microdélétion 22q11.2.
Van L, Heung T, Reyes NGD, Boot E, Chow EWC, Corral M, Bassett AS. Van L, et al. Can J Psychiatry. 2025 Mar;70(3):160-170. doi: 10.1177/07067437241293983. Epub 2024 Dec 6. Can J Psychiatry. 2025. PMID: 39641288 Free PMC article. - Olanzapine versus other atypical antipsychotics for schizophrenia.
Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, Duggan L, Kissling W, Leucht S. Komossa K, et al. Cochrane Database Syst Rev. 2010 Mar 17;2010(3):CD006654. doi: 10.1002/14651858.CD006654.pub2. Cochrane Database Syst Rev. 2010. PMID: 20238348 Free PMC article. Review. - Trace amine-associated receptor 1 (TAAR1) agonism for psychosis: a living systematic review and meta-analysis of human and non-human data.
Siafis S, Chiocchia V, Macleod MR, Austin C, Homiar A, Tinsdeall F, Friedrich C, Ramage FJ, Kennett J, Nomura N, Maksym O, Rutigliano G, Vano LJ, McCutcheon RA, Gilbert D, Ostinelli EG, Stansfield C, Dehdarirad H, Juma DO, Wright S, Simple O, Elugbadebo O, Tonia T, Mantas I, Howes OD, Furukawa TA, Milligan L, Moreno C, Elliott JH, Hastings J, Thomas J, Michie S, Sena ES, Seedat S, Egger M, Potts J, Cipriani A, Salanti G, Leucht S. Siafis S, et al. Wellcome Open Res. 2024 Apr 11;9:182. doi: 10.12688/wellcomeopenres.21302.1. eCollection 2024. Wellcome Open Res. 2024. PMID: 39036710 Free PMC article. - Clinical Correlates of Antipsychotic Plasma Levels with Long-Acting Paliperidone: Corrélats cliniques des concentrations plasmiques de palipéridone à libération prolongée.
Zipursky RB, Agid O, Kiang M, Remington G. Zipursky RB, et al. Can J Psychiatry. 2025 Mar;70(3):209-216. doi: 10.1177/07067437241295648. Epub 2024 Nov 15. Can J Psychiatry. 2025. PMID: 39544022 Free PMC article. - Haloperidol (oral) versus olanzapine (oral) for people with schizophrenia and schizophrenia-spectrum disorders.
Ibragimov K, Keane GP, Carreño Glaría C, Cheng J, Llosa AE. Ibragimov K, et al. Cochrane Database Syst Rev. 2024 Jul 3;7(7):CD013425. doi: 10.1002/14651858.CD013425.pub2. Cochrane Database Syst Rev. 2024. PMID: 38958149 Review.
Cited by
- Recent advances in quantitative neuroproteomics.
Craft GE, Chen A, Nairn AC. Craft GE, et al. Methods. 2013 Jun 15;61(3):186-218. doi: 10.1016/j.ymeth.2013.04.008. Epub 2013 Apr 25. Methods. 2013. PMID: 23623823 Free PMC article. Review. - Metabolic complications of psychotropic medications in psychiatric disorders: Emerging role of de novo lipogenesis and therapeutic consideration.
Khan MM, Khan ZA, Khan MA. Khan MM, et al. World J Psychiatry. 2024 Jun 19;14(6):767-783. doi: 10.5498/wjp.v14.i6.767. eCollection 2024 Jun 19. World J Psychiatry. 2024. PMID: 38984346 Free PMC article. Review. - Preliminary Safety Assessment of New Azinesulfonamide Analogs of Aripiprazole using Prokaryotic Models.
Powroźnik B, Słoczyńska K, Marciniec K, Zajdel P, Pękala E. Powroźnik B, et al. Adv Pharm Bull. 2016 Sep;6(3):377-384. doi: 10.15171/apb.2016.049. Epub 2016 Sep 25. Adv Pharm Bull. 2016. PMID: 27766221 Free PMC article. - Dopamine D(2) receptor function is compromised in the brain of the methionine sulfoxide reductase A knockout mouse.
Oien DB, Ortiz AN, Rittel AG, Dobrowsky RT, Johnson MA, Levant B, Fowler SC, Moskovitz J. Oien DB, et al. J Neurochem. 2010 Jul;114(1):51-61. doi: 10.1111/j.1471-4159.2010.06721.x. Epub 2010 Mar 31. J Neurochem. 2010. PMID: 20374422 Free PMC article. - Olanzapine Reverses MK-801-Induced Cognitive Deficits and Region-Specific Alterations of NMDA Receptor Subunits.
Liu X, Li J, Guo C, Wang H, Sun Y, Wang H, Su YA, Li K, Si T. Liu X, et al. Front Behav Neurosci. 2018 Jan 9;11:260. doi: 10.3389/fnbeh.2017.00260. eCollection 2017. Front Behav Neurosci. 2018. PMID: 29375333 Free PMC article.
References
- Abekawa T, Ito K, Koyama T. Role of the simultaneous enhancement ofNMDA and dopamine D1 receptor-mediated neurotransmission in the effects of clozapine on phencyclidine-induced acute increases in glutamate levels in the rat medial prefrontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:177–193. - PubMed
- Abi-Dargham A. Alterations of serotonin transmission in schizophrenia. Int Rev Neurobiol. 2007;78:133–164. - PubMed
- Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur Psychiatry. 2005;20:15–27. - PubMed
- Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9:404–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical