Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses - PubMed (original) (raw)
Comparative Study
Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses
Predrag Petrovic et al. J Neurosci. 2008.
Abstract
Reward processing is linked to specific neuromodulatory systems with a dopaminergic contribution to reward learning and motivational drive being well established. Neuromodulatory influences on hedonic responses to actual receipt of reward, or punishment, referred to as experienced utility are less well characterized, although a link to the endogenous opioid system is suggested. Here, in a combined functional magnetic resonance imaging-psychopharmacological investigation, we used naloxone to block central opioid function while subjects performed a gambling task associated with rewards and losses of different magnitudes, in which the mean expected value was always zero. A graded influence of naloxone on reward outcome was evident in an attenuation of pleasure ratings for larger reward outcomes, an effect mirrored in attenuation of brain activity to increasing reward magnitude in rostral anterior cingulate cortex. A more striking effect was seen for losses such that under naloxone all levels of negative outcome were rated as more unpleasant. This hedonic effect was associated with enhanced activity in anterior insula and caudal anterior cingulate cortex, areas implicated in aversive processing. Our data indicate that a central opioid system contributes to both reward and loss processing in humans and directly modulates the hedonic experience of outcomes.
Figures
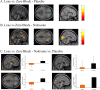
Figure 1.
A, Gambling task 1. Subjects had to choose a left or right wheel of fortune pressing a right or a left key. Each gamble had the same possible outcomes with an expected value of £0. The win and loss outcomes in each gamble varied from £1 to £10, making it possible to measure responses related to outcome magnitude. Subjects rated how they experienced the outcome of each gamble on a visual analog scale (VAS) from −100 (highest imaginable unpleasantness) to 100 (highest imaginable pleasantness). B, C, Behavioral results. The ratings of the subjects for all outcomes in the placebo and the naloxone day (B). Although treatment did not show any significant difference for overall reward outcomes, a significant effect was observed for the losses in that losses were experienced as more unpleasant after naloxone treatment with 10 of the 13 subjects showing this effect (C).
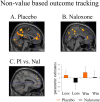
Figure 2.
Loss processing under placebo and naloxone. A, For placebo, loss versus zero outcome block showed increased activity in midcaudal and rostral ACC, bilateral insula, and visual cortex/precuneus. B, The same areas were activated in the naloxone condition. C, Testing for an interaction (including the parameter estimates) indicated greater activation for loss versus zero outcome blocks in caudal ACC, bilateral insula, subgenual ACC, precuneus, and extrastriate visual cortex under naloxone compared with placebo treatment. Error bars indicate SEM.
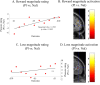
Figure 3.
Naloxone effect on outcome magnitude. A, B, Activity in rACC and PCC correlated with the outcome magnitude independent of value (from £1 to £10 for both rewards and losses) for both placebo (A) and naloxone (B) treatment. C, The effect was attenuated in rACC after naloxone treatment and was evident both for losses and rewards. Error bars indicate SEM. Pl, Placebo; Nal, naloxone.
Figure 4.
Naloxone effect on outcome magnitude for pleasure ratings and rACC activity in rewards and losses. A, B, Naloxone had a more negative effect the larger the reward outcome magnitude was both for affective ratings and activity in rACC. A, For the behavioral ratings, this was manifest in a positive correlation between the treatment difference [placebo (Pl) vs naloxone (Nal)] in hedonic ratings for each reward outcome (_y_-axis) and the outcome (_x_-axis) (Spearman's ρ: r = 0.728; p < 0.005). Thus, larger outcome magnitudes were experienced as less pleasurable under naloxone compared with placebo. This effect was mirrored by a reward outcome magnitude-related activity in rACC after placebo treatment that was attenuated after naloxone treatment (B). C, D, Although no significant behavioral effects of treatment were observed for the different magnitudes in losses (C), a similar effect was observed in the rACC (D). Here, the outcome magnitude of loss was attenuated after naloxone treatment. Thus, the correlation between activity in rACC and loss outcome magnitude (£1–£10) was suppressed by naloxone. Note that the observed magnitude effect in rACC was independent of positive or negative value.
Similar articles
- Context-dependent cortical activation in response to financial reward and penalty: an event-related fMRI study.
Akitsuki Y, Sugiura M, Watanabe J, Yamashita K, Sassa Y, Awata S, Matsuoka H, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Akitsuki Y, et al. Neuroimage. 2003 Aug;19(4):1674-85. doi: 10.1016/s1053-8119(03)00250-7. Neuroimage. 2003. PMID: 12948722 - Dissociation between the processing of humorous and monetary rewards in the 'motivation' and 'hedonic' brains.
Chan YC, Hsu WC, Chou TL. Chan YC, et al. Sci Rep. 2018 Oct 18;8(1):15425. doi: 10.1038/s41598-018-33623-4. Sci Rep. 2018. PMID: 30337614 Free PMC article. - Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry.
Clark L, Lawrence AJ, Astley-Jones F, Gray N. Clark L, et al. Neuron. 2009 Feb 12;61(3):481-90. doi: 10.1016/j.neuron.2008.12.031. Neuron. 2009. PMID: 19217383 Free PMC article. - Contributions of anterior cingulate cortex to behaviour.
Devinsky O, Morrell MJ, Vogt BA. Devinsky O, et al. Brain. 1995 Feb;118 ( Pt 1):279-306. doi: 10.1093/brain/118.1.279. Brain. 1995. PMID: 7895011 Review. - The physiology of opiate hedonic effects and the role of opioids in motivated behavior.
Carr KD. Carr KD. Adv Alcohol Subst Abuse. 1984 Spring;3(3):5-18. doi: 10.1300/J251v03n03_02. Adv Alcohol Subst Abuse. 1984. PMID: 6388274 Review.
Cited by
- Brain imaging studies in pathological gambling.
van Holst RJ, van den Brink W, Veltman DJ, Goudriaan AE. van Holst RJ, et al. Curr Psychiatry Rep. 2010 Oct;12(5):418-25. doi: 10.1007/s11920-010-0141-7. Curr Psychiatry Rep. 2010. PMID: 20676945 Free PMC article. Review. - Enhanced incentive motivation for sucrose-paired cues in adolescent rats: possible roles for dopamine and opioid systems.
Burton CL, Noble K, Fletcher PJ. Burton CL, et al. Neuropsychopharmacology. 2011 Jul;36(8):1631-43. doi: 10.1038/npp.2011.44. Epub 2011 Apr 20. Neuropsychopharmacology. 2011. PMID: 21508935 Free PMC article. - Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies.
Liu X, Hairston J, Schrier M, Fan J. Liu X, et al. Neurosci Biobehav Rev. 2011 Apr;35(5):1219-36. doi: 10.1016/j.neubiorev.2010.12.012. Epub 2010 Dec 24. Neurosci Biobehav Rev. 2011. PMID: 21185861 Free PMC article. Review. - Functional magnetic resonance imaging of internet addiction in young adults.
Sepede G, Tavino M, Santacroce R, Fiori F, Salerno RM, Di Giannantonio M. Sepede G, et al. World J Radiol. 2016 Feb 28;8(2):210-25. doi: 10.4329/wjr.v8.i2.210. World J Radiol. 2016. PMID: 26981230 Free PMC article. - Sharing pain and relief: neural correlates of physicians during treatment of patients.
Jensen KB, Petrovic P, Kerr CE, Kirsch I, Raicek J, Cheetham A, Spaeth R, Cook A, Gollub RL, Kong J, Kaptchuk TJ. Jensen KB, et al. Mol Psychiatry. 2014 Mar;19(3):392-8. doi: 10.1038/mp.2012.195. Epub 2013 Jan 29. Mol Psychiatry. 2014. PMID: 23358155 Free PMC article.
References
- Arntz A, Merckelbach H, de Jong P. Opioid antagonist affects behavioral effects of exposure in vivo. J Consult Clin Psychol. 1993;61:865–870. - PubMed
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. - PubMed
- Bentham J. An introduction to the principles of morals and legislation. Oxford: Clarendon; 1907.
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. - PubMed
- Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources