The molecular neurobiology of depression - PubMed (original) (raw)
Review
The molecular neurobiology of depression
Vaishnav Krishnan et al. Nature. 2008.
Abstract
Unravelling the pathophysiology of depression is a unique challenge. Not only are depressive syndromes heterogeneous and their aetiologies diverse, but symptoms such as guilt and suicidality are impossible to reproduce in animal models. Nevertheless, other symptoms have been accurately modelled, and these, together with clinical data, are providing insight into the neurobiology of depression. Recent studies combining behavioural, molecular and electrophysiological techniques reveal that certain aspects of depression result from maladaptive stress-induced neuroplastic changes in specific neural circuits. They also show that understanding the mechanisms of resilience to stress offers a crucial new dimension for the development of fundamentally novel antidepressant treatments.
Conflict of interest statement
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at www.nature.com/nature.
Figures
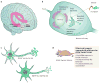
Figure 1. Neural circuitry of depression
Several brain regions are implicated in the pathophysiology of depression. a, Deep brain stimulation of the subgenual cingulate cortex (Cg25) or the nucleus accumbens (NAc) has an antidepressant effect on individuals who have treatment-resistant depression. This effect is thought to be mediated through inhibiting the activity of these regions either by depolarization blockade or by stimulation of passing axonal fibres. (Image courtesy of T. Schlaepfer and V. Sturm, University Hospital, Bonn, Germany.) b, Increased activity-dependent release of brain-derived neurotrophic factor (BDNF) within the mesolimbic dopamine circuit (dopamine-producing ventral tegmental area (VTA) to dopamine-sensitive NAc) mediates susceptibility to social stress, probably occurring in part through activation of the transcription factor CREB (cyclic-AMP-response-element-binding protein) by phosphorylation (P). c, Neuroimaging studies strongly implicate the amygdala (red pixels show activated areas) as an important limbic node for processing emotionally salient stimuli, such as fearful faces. (Image courtesy of D. Weinberger, National Institute of Mental Health, Bethesda, Maryland). d, Stress decreases the concentrations of neurotrophins (such as BDNF), the extent of neurogenesis and the complexity of neuronal processes in the hippocampus (HP), effects that are mediated in part through increased cortisol concentrations and decreased CREB activity ,. e, Peripherally released metabolic hormones in addition to cortisol, such as ghrelin and leptin, produce mood-related changes through their effects on the hypothalamus (HYP) and several limbic regions (for example, the hippocampus, VTA and NAc). DR, dorsal raphe; LC, locus coeruleus; PFC, prefrontal cortex.
Figure 2. BDNF and depression — an example of the complexities of the molecular pathophysiology of depression
a, Post-mortem data from depressed humans show that depression is associated with a decrease in the amount of BDNF in the hippocampus and an increase (of similar magnitude) in the NAc, an example of the regional specificity of depression-related neuroplastic changes. b, Neuronal secretion of BDNF occurs through regulated (activity-dependent) and constitutive secretory pathways. Regulated secretion is modulated by the interactions of proteins in the Golgi apparatus with the pro-domain of BDNF, the site of a single-nucleotide polymorphism (G196A) in humans that results in the substitution of valine at amino-acid residue 66 with methionine. c, The Met-66-containing BDNF variant has impaired intracellular trafficking. Met-66 BDNF is not properly sorted within the cell, causing it to be distributed throughout the cell body outside of vesicles. In addition, less BDNF is secreted from the nerve terminal. d, Knock-in mice that homozygously express Met-66 BDNF have normal responses in the forced-swim test, but these mice show more anxiety-like behaviour and greater resilience to behavioural and molecular changes after social defeat, implicating this BDNF polymorphism in the pathophysiology of psychological disorders that are influenced by stressful life events.
Figure 3. Epigenetic regulation in depression
The transcriptional potential of genes involved in neuroplastic responses to stress or antidepressant treatments can be regulated through chromatin-remodelling events catalysed by specific enzymes. a, The methylation of histones on specific lysine residues (for example, Lys 9 and Lys 27) is associated with condensed chromatin (heterochromatin) and is important in the repression of Bdnf expression in the hippocampus after social defeat. The pluses and minuses indicate activation or inhibition, respectively, of a particular process. b, By contrast, repression of other genes can occur through the methylation of cytosine within CpG islands of promoter regions, attracting proteins involved in transcriptional repression, such as SIN3A, MeCP2 (methyl-CpG binding protein 2) and histone deacetylases (HDACs). DNA methylation of the promoter of the glucocorticoid receptor gene occurs in rat pups born to mothers with inherently low levels of maternal behaviour. Although such methylation events have been reported to be reversible, the enzymes responsible for demethylating DNA have yet to be identified,. c, Histone acetylation, catalysed by histone acetyltransferases, is associated with decondensed chromatin (euchromatin), increasing the activity of transcriptional complexes. HDAC inhibitors (which activate the expression of numerous genes that have not yet been identified with certainty) show antidepressant properties in several assays,. Ac, acetyl; Me, methyl.
Similar articles
- Effects of genes and stress on the neurobiology of depression.
Mann JJ, Currier D. Mann JJ, et al. Int Rev Neurobiol. 2006;73:153-89. doi: 10.1016/S0074-7742(06)73005-7. Int Rev Neurobiol. 2006. PMID: 16737904 Review. No abstract available. - [Molecular and cellular mechanisms of depression. Role of glucocorticoids, cytokines, neurotransmitters, and trophic factors in genesis of depressive disorders].
Grigor'ian GA, Dygalo NN, Gekht AB, Stepanichev MIu, Guliaeva NV. Grigor'ian GA, et al. Usp Fiziol Nauk. 2014 Apr-Jun;45(2):3-19. Usp Fiziol Nauk. 2014. PMID: 25707260 Review. Russian. - [Recent progress in neurobiological mechanisms of depression].
Gao YB, Li LP, Zhu XH, Gao TM. Gao YB, et al. Sheng Li Xue Bao. 2012 Aug 25;64(4):475-80. Sheng Li Xue Bao. 2012. PMID: 22907310 Review. Chinese. - Neurodevelopmental origins of depressive disorders.
Ansorge MS, Hen R, Gingrich JA. Ansorge MS, et al. Curr Opin Pharmacol. 2007 Feb;7(1):8-17. doi: 10.1016/j.coph.2006.11.006. Epub 2006 Dec 21. Curr Opin Pharmacol. 2007. PMID: 17188022 Review. - [The neurobiology of depression].
Haffen E, Sechter D. Haffen E, et al. Rev Prat. 1999 Apr 1;49(7):707-12. Rev Prat. 1999. PMID: 10337213 French.
Cited by
- Are Essential Trace Elements Effective in Modulation of Mental Disorders? Update and Perspectives.
Shayganfard M. Shayganfard M. Biol Trace Elem Res. 2022 Mar;200(3):1032-1059. doi: 10.1007/s12011-021-02733-y. Epub 2021 Apr 27. Biol Trace Elem Res. 2022. PMID: 33904124 Review. - Hypothalamic Proteomic Analysis Reveals Dysregulation of Glutamate Balance and Energy Metabolism in a Mouse Model of Chronic Mild Stress-Induced Depression.
Rao C, Shi H, Zhou C, Zhu D, Zhao M, Wang Z, Yang Y, Chen J, Liao L, Tang J, Wu Y, Zhou J, Cheng K, Xie P. Rao C, et al. Neurochem Res. 2016 Sep;41(9):2443-56. doi: 10.1007/s11064-016-1957-2. Epub 2016 May 26. Neurochem Res. 2016. PMID: 27230881 - Desipramine protects neuronal cell death and induces heme oxygenase-1 expression in Mes23.5 dopaminergic neurons.
Lin HY, Yeh WL, Huang BR, Lin C, Lai CH, Lin H, Lu DY. Lin HY, et al. PLoS One. 2012;7(11):e50138. doi: 10.1371/journal.pone.0050138. Epub 2012 Nov 27. PLoS One. 2012. PMID: 23209658 Free PMC article. - The Research Domain Criteria (RDoC) Project and Studies of Risk and Resilience in Maltreated Children.
Kaufman J, Gelernter J, Hudziak JJ, Tyrka AR, Coplan JD. Kaufman J, et al. J Am Acad Child Adolesc Psychiatry. 2015 Aug;54(8):617-25. doi: 10.1016/j.jaac.2015.06.001. Epub 2015 Jun 10. J Am Acad Child Adolesc Psychiatry. 2015. PMID: 26210330 Free PMC article. Review. - Striatal Shati/Nat8l-BDNF pathways determine the sensitivity to social defeat stress in mice through epigenetic regulation.
Miyanishi H, Muramatsu SI, Nitta A. Miyanishi H, et al. Neuropsychopharmacology. 2021 Aug;46(9):1594-1605. doi: 10.1038/s41386-021-01033-2. Epub 2021 Jun 7. Neuropsychopharmacology. 2021. PMID: 34099867 Free PMC article.
References
- Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. - PubMed
- Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. - PubMed
- Knol MJ, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. - PubMed
- Evans DL, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 MH066172-07/MH/NIMH NIH HHS/United States
- R01 MH051399-18/MH/NIMH NIH HHS/United States
- P50 MH066172-06/MH/NIMH NIH HHS/United States
- R01 MH051399-17/MH/NIMH NIH HHS/United States
- P50 MH066172/MH/NIMH NIH HHS/United States
- R01 MH051399/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical