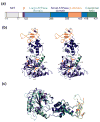Biochemical and structural studies of yeast Vps4 oligomerization - PubMed (original) (raw)
Biochemical and structural studies of yeast Vps4 oligomerization
Malgorzata D Gonciarz et al. J Mol Biol. 2008.
Abstract
The ESCRT (endosomal sorting complexes required for transport) pathway functions in vesicle formation at the multivesicular body, the budding of enveloped RNA viruses such as HIV-1, and the final abscission stage of cytokinesis. As the only known enzyme in the ESCRT pathway, the AAA ATPase (ATPase associated with diverse cellular activities) Vps4 provides the energy required for multiple rounds of vesicle formation. Like other Vps4 proteins, yeast Vps4 cycles through two states: a catalytically inactive disassembled state that we show here is a dimer and a catalytically active higher-order assembly that we have modeled as a dodecamer composed of two stacked hexameric rings. We also report crystal structures of yeast Vps4 proteins in the apo- and ATPgammaS [adenosine 5'-O-(3-thiotriphosphate)]-bound states. In both cases, Vps4 subunits assembled into continuous helices with 6-fold screw axes that are analogous to helices seen previously in other Vps4 crystal forms. The helices are stabilized by extensive interactions between the large and small AAA ATPase domains of adjacent Vps4 subunits, suggesting that these contact surfaces may be used to build both the catalytically active dodecamer and catalytically inactive dimer. Consistent with this model, we have identified interface mutants that specifically inhibit Vps4 dimerization, dodecamerization, or both. Thus, the Vps4 dimer and dodecamer likely form distinct but overlapping interfaces. Finally, our structural studies have allowed us to model the conformation of a conserved loop (pore loop 2) that is predicted to form an arginine-rich pore at the center of one of the Vps4 hexameric rings. Our mutational analyses demonstrate that pore loop 2 residues Arg241 and Arg251 are required for efficient HIV-1 budding, thereby supporting a role for this "arginine collar" in Vps4 function.
Figures
Figure 1
Structure of yeast Vps4. (a) Domain organization of the human and yeast Vps4 proteins. Numbering scheme corresponds to the yeast Vps4 protein. (b) Stereoview of the Vps4ΔMIT structure. This structure corresponds to molecule A in the ATPγS bound state in crystal form 2. Domains are color coded as in part (a). Note that the small helix spanning residues 199–206 is not numbered in order to maintain consistency with previous conventions. (c) Superposition of yeast Vps4ΔMIT (in crystal form 1) and human VPS4BΔMIT (lighter shades) in their sulfate-bound states. The two proteins overlay with an RMSD of 1.2 Å over 225 Cα positions within the AAA ATPase cassette.
Figure 2
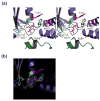
Vps4 nucleotide binding pockets. (a) Stereoview of the Vps4-ATPγS nucleotide binding site of molecule A in crystal form 2. Active site Vps4 residues are color coded according to their functional roles in Mg2+ coordination/ATP hydrolysis (cyan, S180, D232 and E/Q233), adenine ring stacking (magenta, Y181 and M307) and phosphate sensing (yellow, K179 and N277) with the “arginine finger” residue R288 from an adjacent molecule in the modeled hexamer shown in green. Note that the E233Q mutant was used here and throughout to allow ATP/ATPγS binding while inhibiting hydrolysis. (b) Electron density for the ATPγS nucleotides in molecule A of Vps4ΔMIT crystal form 2. The densities show (F0 – FC) omit maps contoured at 2.5 σ.
Figure 3
Interdomain flexibility in different Vps4ΔMIT crystal structures. Superposition of six independent structures of Vps4ΔMIT in complex with: no ligand (2RKO, white), sulfate (crystal form 1, green), phosphate (2QPA, molecule B, blue), ADP (2QPA, molecule A, red), ATPγS (crystal form 2, molecule A, pink), and ATPγS (crystal form 2, molecule C, purple). Superposition on the large ATPase domains reveals that the small ATPase domain can rotate by up to 19° about a hinge angle located in the linker between the two domains and centered at residue Pro297. A second hinge within the small ATPase domain is also evident in the middle of the extended helix α8, centered about residue Pro350. These two hinge angles were defined by first finding the centers of masses of the large ATPase domain (residues 122–298 and 418–433), the core of the small domain (300–350; 402–414), and the β domain (358–399) using the program 6d_moleman2. Hinge angle I was then calculated as the angle between the large and small domains, with Pro297 Cα at the apex, and hinge angle II was calculated as the angle between the small domain and the β domain, with Pro350 Cα at the apex.
Figure 4
Vps4 proteins dimerize in solution Representative equilibrium sedimentation profiles for full length Vps4 (panel 1), Vps4ΔMIT (panel 2), Vps4ΔMIT, Q216A (panel 3, Interface 1 mutant), and Vps4ΔMIT, L151D (panel 4, Interface 2 mutant). Sedimentation data are plotted as absorbance versus the distance from the center of the axis of rotation (radius). To simplify the plot, the radius was normalized so that the data from all three sectors (i.e. three different concentrations) overlap. In each case, data from three protein concentrations and one speed are displayed (open symbols) along with the best single ideal species fit (solid lines). The best fits for each protein were derived from global fits to data collected at three concentrations and two speeds. The residuals (differences between the raw absorbance data and the fit are shown below each panel.
Figure 5
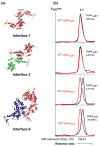
Mutational analyses of crystallographic Vps4 dimer interfaces. (a) Crystallographic Vps4 dimer interfaces. Interface 1 is a symmetric interface between two large ATPase domains (residue Q216 is shown in cyan), Interface 2 is a symmetric interface between two small ATPase domains (residue L407 is shown in blue), and Interface 6 is an asymmetric interface between the large and small ATPase domains (residues L151 and W388 are shown in orange and green, respectively). (b) Gel filtration chromatograms of Vps4ΔMIT proteins with the following mutations: Q216A (Interface 1), L407D (Interface 2), L151D (Interface 6), and W388A (Interface 6). Vps4ΔMIT proteins used here and elsewhere contained the E233Q mutation, which allowed ATP binding but inhibited hydrolysis. For reference, the elution profile of the “wild type” Vps4ΔMIT, E233Q protein is shown in red in each panel, elution positions for monomeric (1) and dimeric (2) proteins are shown as dotted vertical lines, and the elution positions of molecular weight standards are shown below the chromatograms. Vps4 protein concentrations were 150 μM in all cases. Note that at low micromolar concentrations, the dimeric proteins exhibited concentration-dependent mobilities (not shown), indicating that appreciable concentrations of monomers could accumulate under these low protein and non-equilibrium conditions.
Figure 6
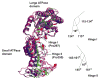
Mutagenesis test of the Vps4ΔMIT hexamer homology model. (a) The “p97 D1 Homology” model for a Vps4ΔMIT hexameric ring within the dodecameric enzyme (upper) is shown together with the arrangement of Vps4 along the crystallographic six-fold screw axis (lower). The p97 D1 Homology Model was created by superimposing Vps4ΔMIT subunits onto the crystal structure of the hexameric ring of the homologous p97 D1 protein. The Trp388 (green) and Leu151 (orange) residues are shown explicitly in homology model and crystal structure to illustrate how the Leu151 residue contributes to the interface in both models whereas the Trp388 residue contributes to the interface in the crystal structure but not in the p97 D1 Homology Model (see insets). (b) Gel filtration chromatograms of wild type and mutant Vps4ΔMIT proteins in the absence (black) or presence (green) of ATP. For reference, elution positions for monomeric (1), dimeric (2), and dodecameric (12) proteins are shown as dotted vertical lines, and the elution positions of molecular weight standards are shown below the chromatograms. Note that upon addition of ATP, the wild type Vps4ΔMIT protein converts from a dimer to a dodecamer, Vps4ΔMIT, L151 remains a monomer, and Vps4ΔMIT, W388A converts from a monomer to a dodecamer.
Figure 7
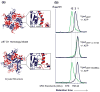
VPS4A Pore Loop 2 residues are required for HIV budding. (a) Two views of the p97 D1 Homology model for a hexameric ring of yeast Vps4ΔMIT in the ATPγS-bound state. Pore loop 1 residues (206WMG208) are shown in green, and a subset of the charged Pore Loop 2 residues are shown in blue (basic residues) or red (acidic residues). (b) Sequence alignments highlighting Pore Loop 1 (green) and Pore Loop 2 (blue, basic, and red, acidic) residues from human and yeast VPS4 proteins. Equivalent residues from the related p97 and Spastin AAA ATPase cassettes are highlighted following the same color scheme (except for non-conservative changes). (c) Vps4 Pore Loop 2 mutations dominantly inhibit HIV-1 release and infectivity. 293T cells were co-transfected with an empty vector control (lane 1) or with a proviral HIV-1 expression vector (lanes 2–7) together with the designated GFP-VPS4A expression constructs. The top two western blots show the levels of virion-associated CA and MA proteins released into the supernatant (Panel 1, Virion) or expressed in the cells (Panel 2, Cell). The bottom two western blots show cellular levels of GFP-VPS4A and of an γ-Tubulin control. The bottom panel shows vector titers in the supernatants under the different conditions (infectious units/ml). Note that expression of the different Pore Loop 2 mutant GFP-VPS4A constructs (lanes 3–6) or of the VPS4A ATPase mutant (lane 7, positive control) dominantly inhibited virion release and titers. Infectivity values are the mean of measurements from quadruplicate repeats and error bars indicate standard deviations.
Similar articles
- The oligomeric state of the active Vps4 AAA ATPase.
Monroe N, Han H, Gonciarz MD, Eckert DM, Karren MA, Whitby FG, Sundquist WI, Hill CP. Monroe N, et al. J Mol Biol. 2014 Feb 6;426(3):510-25. doi: 10.1016/j.jmb.2013.09.043. Epub 2013 Oct 23. J Mol Biol. 2014. PMID: 24161953 Free PMC article. - Binding of Substrates to the Central Pore of the Vps4 ATPase Is Autoinhibited by the Microtubule Interacting and Trafficking (MIT) Domain and Activated by MIT Interacting Motifs (MIMs).
Han H, Monroe N, Votteler J, Shakya B, Sundquist WI, Hill CP. Han H, et al. J Biol Chem. 2015 May 22;290(21):13490-9. doi: 10.1074/jbc.M115.642355. Epub 2015 Apr 1. J Biol Chem. 2015. PMID: 25833946 Free PMC article. - Structural characterization of the ATPase reaction cycle of endosomal AAA protein Vps4.
Xiao J, Xia H, Yoshino-Koh K, Zhou J, Xu Z. Xiao J, et al. J Mol Biol. 2007 Nov 30;374(3):655-70. doi: 10.1016/j.jmb.2007.09.067. Epub 2007 Sep 29. J Mol Biol. 2007. PMID: 17949747 Free PMC article. - Structures, Functions, and Dynamics of ESCRT-III/Vps4 Membrane Remodeling and Fission Complexes.
McCullough J, Frost A, Sundquist WI. McCullough J, et al. Annu Rev Cell Dev Biol. 2018 Oct 6;34:85-109. doi: 10.1146/annurev-cellbio-100616-060600. Epub 2018 Aug 10. Annu Rev Cell Dev Biol. 2018. PMID: 30095293 Free PMC article. Review. - Meiotic Clade AAA ATPases: Protein Polymer Disassembly Machines.
Monroe N, Hill CP. Monroe N, et al. J Mol Biol. 2016 May 8;428(9 Pt B):1897-911. doi: 10.1016/j.jmb.2015.11.004. Epub 2015 Nov 10. J Mol Biol. 2016. PMID: 26555750 Free PMC article. Review.
Cited by
- Membrane budding and scission by the ESCRT machinery: it's all in the neck.
Hurley JH, Hanson PI. Hurley JH, et al. Nat Rev Mol Cell Biol. 2010 Aug;11(8):556-66. doi: 10.1038/nrm2937. Epub 2010 Jun 30. Nat Rev Mol Cell Biol. 2010. PMID: 20588296 Free PMC article. Review. - Vfa1 binds to the N-terminal microtubule-interacting and trafficking (MIT) domain of Vps4 and stimulates its ATPase activity.
Vild CJ, Xu Z. Vild CJ, et al. J Biol Chem. 2014 Apr 11;289(15):10378-10386. doi: 10.1074/jbc.M113.532960. Epub 2014 Feb 24. J Biol Chem. 2014. PMID: 24567329 Free PMC article. - Live-cell visualization of dynamics of HIV budding site interactions with an ESCRT component.
Baumgärtel V, Ivanchenko S, Dupont A, Sergeev M, Wiseman PW, Kräusslich HG, Bräuchle C, Müller B, Lamb DC. Baumgärtel V, et al. Nat Cell Biol. 2011 Apr;13(4):469-74. doi: 10.1038/ncb2215. Epub 2011 Mar 10. Nat Cell Biol. 2011. PMID: 21394086 - Mechanism of Vps4 hexamer function revealed by cryo-EM.
Su M, Guo EZ, Ding X, Li Y, Tarrasch JT, Brooks CL 3rd, Xu Z, Skiniotis G. Su M, et al. Sci Adv. 2017 Apr 14;3(4):e1700325. doi: 10.1126/sciadv.1700325. eCollection 2017 Apr. Sci Adv. 2017. PMID: 28439563 Free PMC article. - Asymmetric ring structure of Vps4 required for ESCRT-III disassembly.
Caillat C, Macheboeuf P, Wu Y, McCarthy AA, Boeri-Erba E, Effantin G, Göttlinger HG, Weissenhorn W, Renesto P. Caillat C, et al. Nat Commun. 2015 Dec 3;6:8781. doi: 10.1038/ncomms9781. Nat Commun. 2015. PMID: 26632262 Free PMC article.
References
- Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32:561–73. - PubMed
- Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–68. - PubMed
- Welsch S, Habermann A, Jager S, Muller B, Krijnse-Locker J, Krausslich HG. Ultrastructural analysis of ESCRT proteins suggests a role for endosome-associated tubular-vesicular membranes in ESCRT function. Traffic. 2006;7:1551–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 GM082545/GM/NIGMS NIH HHS/United States
- R01 AI051174/AI/NIAID NIH HHS/United States
- R37 AI051174/AI/NIAID NIH HHS/United States
- P50 GM082545-029003/GM/NIGMS NIH HHS/United States
- P50 GM082545-02/GM/NIGMS NIH HHS/United States
- R01 AI051174-07/AI/NIAID NIH HHS/United States
- AI51174/AI/NIAID NIH HHS/United States
- P50 GM082545-029002/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials