TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements - PubMed (original) (raw)
. 2009 Jan 8;113(2):309-16.
doi: 10.1182/blood-2008-07-166421. Epub 2008 Oct 17.
Elena Pumbo, Jennifer Ivanovich, Ping An, Richard T Maziarz, Ulrike M Reiss, Deborah Chirnomas, Akiko Shimamura, Adrianna Vlachos, Jeffrey M Lipton, Rakesh K Goyal, Frederick Goldman, David B Wilson, Philip J Mason, Monica Bessler
Affiliations
- PMID: 18931339
- PMCID: PMC2615648
- DOI: 10.1182/blood-2008-07-166421
TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements
Hong-Yan Du et al. Blood. 2009.
Abstract
Dyskeratosis congenita (DC) is a rare inherited form of bone marrow failure (BMF) caused by mutations in telomere maintaining genes including TERC and TERT. Here we studied the prevalence of TERC and TERT gene mutations and of telomere shortening in an unselected population of patients with BMF at our medical center and in a selected group of patients referred from outside institutions. Less than 5% of patients with BMF had pathogenic mutations in TERC or TERT. In patients with BMF, pathogenic TERC or TERT gene mutations were invariably associated with marked telomere shortening (<< 1st percentile) in peripheral blood mononuclear cells (PBMCs). In asymptomatic family members, however, telomere length was not a reliable predictor for the presence or absence of a TERC or TERT gene mutation. Telomere shortening was not pathognomonic of DC, as approximately 30% of patients with BMF due to other causes had PBMC telomere lengths at the 1st percentile or lower. We conclude that in the setting of BMF, measurement of telomere length is a sensitive but nonspecific screening method for DC. In the absence of BMF, telomere length measurements should be interpreted with caution.
Figures
Figure 1
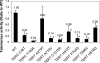
In vitro telomerase activity of the TERC RNA and TERT variants in WI-38 VA-13 cells. WI-38 VA-13 cells were transfected with a plasmid expressing the mutant or WT TERT cDNA sequences and a plasmid expressing the respective WT or mutant or TERC RNA. Telomerase activity was determined using a quantitative PCR-based TRAP assay. Activity is shown in comparison to the activity obtained after transfection with WT (= 1.0). Values above each column are the average of triplicate experiments. Error bars represent SD, * indicates the difference between the variant, and WT is statistically significant at the level of .05. In vitro telomerase activities of the TERT mutants H412Y, P704S, Y846C, and H876Q have been reported previously.
Figure 2
Telomere lengths in PBMC from patients with BMF. Telomere lengths in PBMC were measured by flow cytometric fluorescence in situ hybridization. (A) Telomere length in 234 healthy control subjects between the ages of 1 day and 94 years. The 1st, 5th, 25th, 50th, 75th, 95th, 99th percentiles of healthy controls are shown. (B) Telomere length in 160 patients with BMF from whom telomere measurements were obtained. (C) Telomere length in patients enrolled in the study with the diagnosis of DC. The red squares highlight patients with DC, while gray circles represent the remaining patients with BMF. (D) Telomere lengths in patients with DBA (highlighted in blue). (E) Telomere length in patients with SDS (highlighted in pink). (F) Telomere length in patients with MDS (highlighted in green). Arrows indicate patients in whom a pathogenic C35T TERC gene mutation was identified. (G) Telomere length in patients with PNH (highlighted in brown). (H) Telomere length in patients with aplastic anemia not otherwise classified (AA, highlighted in purple). Arrows indicate patients in whom a pathogenic TERT gene mutation was identified (from left to right; Y846C/H876Q, A716V, A716V). Stippled arrows indicate patients with the variant H412Y. (I) Telomere length in patients with other forms of IBMFS (Pearson syndrome, Fanconi anemia, and FPD, from left to right, highlighted in gold).
Figure 3
Telomere lengths in patients with DC and first-degree family members. (A) Patients with DC due to a TERC gene mutation. (B) Patients with DC due to a TERT gene mutation. (C) Patients with DC due to a DKC1 gene mutation. People with signs of BMF are shown in black; family members without BMF are shown in gray. Heterozygous mutation carriers are indicated with a half-filled symbol; filled symbol indicates homozygosity (hemizygosity/compound heterozygosity) for the mutation. (D) Telomere length in 41 subjects with a de novo TERT gene deletion due to a chromosome deletion syndrome that includes the TERT gene (5p- syndrome).
Similar articles
- TERT and TERC mutations detected in cryptic dyskeratosis congenita suppress telomerase activity.
Terada K, Miyake K, Yamaguchi H, Miyake N, Yamanaka K, Kojima S, Ito E, Inokuchi K, Okada T. Terada K, et al. Int J Lab Hematol. 2020 Jun;42(3):316-321. doi: 10.1111/ijlh.13176. Epub 2020 Mar 9. Int J Lab Hematol. 2020. PMID: 32150348 - TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes.
Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. Walne AJ, et al. Blood. 2008 Nov 1;112(9):3594-600. doi: 10.1182/blood-2008-05-153445. Epub 2008 Jul 30. Blood. 2008. PMID: 18669893 Free PMC article. - Evaluation and Management of Hematopoietic Failure in Dyskeratosis Congenita.
Agarwal S. Agarwal S. Hematol Oncol Clin North Am. 2018 Aug;32(4):669-685. doi: 10.1016/j.hoc.2018.04.003. Epub 2018 May 28. Hematol Oncol Clin North Am. 2018. PMID: 30047419 Free PMC article. Review. - Dyskeratosis congenita, stem cells and telomeres.
Kirwan M, Dokal I. Kirwan M, et al. Biochim Biophys Acta. 2009 Apr;1792(4):371-9. doi: 10.1016/j.bbadis.2009.01.010. Epub 2009 Feb 7. Biochim Biophys Acta. 2009. PMID: 19419704 Free PMC article. Review.
Cited by
- Tert-expressing cells contribute to salivary gland homeostasis and tissue regeneration after radiation therapy.
Guan L, Viswanathan V, Jiang Y, Vijayakumar S, Cao H, Zhao J, Colburg DRC, Neuhöfer P, Zhang Y, Wang J, Xu Y, Laseinde EE, Hildebrand R, Rahman M, Frock R, Kong C, Beachy PA, Artandi S, Le QT. Guan L, et al. Genes Dev. 2024 Jul 19;38(11-12):569-582. doi: 10.1101/gad.351577.124. Genes Dev. 2024. PMID: 38997156 - 2.7 Å cryo-EM structure of human telomerase H/ACA ribonucleoprotein.
Ghanim GE, Sekne Z, Balch S, van Roon AM, Nguyen THD. Ghanim GE, et al. Nat Commun. 2024 Jan 25;15(1):746. doi: 10.1038/s41467-024-45002-x. Nat Commun. 2024. PMID: 38272871 Free PMC article. - Telomeres: Dysfunction, Maintenance, Aging and Cancer.
Liao P, Yan B, Wang C, Lei P. Liao P, et al. Aging Dis. 2023 Nov 29;15(6):2595-2631. doi: 10.14336/AD.2023.1128. Aging Dis. 2023. PMID: 38270117 Review. - Studying the pathogenicity of 26 variants characterized in the first molecular analyses of Egyptian aplastic anemia patients.
Sokkar MF, Hamdy M, Erian PS, Mosaad RM, Elaraby NM, Taher MB, El-Sayed H, Al Komy M, Eid MM, Mohamed AM, Amr KS, El-Kamah GY. Sokkar MF, et al. J Genet Eng Biotechnol. 2023 Nov 29;21(1):149. doi: 10.1186/s43141-023-00585-8. J Genet Eng Biotechnol. 2023. PMID: 38017244 Free PMC article. - Oncogene SCARNA12 as a potential diagnostic biomarker for colorectal cancer.
Zhang H, Liu X, Zhang W, Deng J, Lin C, Qi Z, Li Y, Gu Y, Wang Q, Shen L, Wang Z. Zhang H, et al. Mol Biomed. 2023 Nov 1;4(1):37. doi: 10.1186/s43556-023-00147-x. Mol Biomed. 2023. PMID: 37907779 Free PMC article.
References
- Cole H, Rauschkolb J, Toomey J. Dyskeratosis congenita with pigmentation, dystrophia unguis and leukokeratosis oris. Arch Dermatol Syphiligr. 1930;21:71–95. - PubMed
- Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. - PubMed
- Bessler M, Mason PJ, Link DC, Wilson DB. Inherited bone marrow failure syndromes. In: Nathan DG, Oski FA, editors. Hematology of Infancy and Childhood. (7th ed) Vol. 1. Philadelphia, PA: W.B. Saunders; in press.
- Heiss NS, Knight SW, Vulliamy TJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions ‘see comments’. Nat Genet. 1998;19:32–38. - PubMed
- Vulliamy T, Marrone A, Goldman F, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA105312/CA/NCI NIH HHS/United States
- CA106995/CA/NCI NIH HHS/United States
- R01 CA106995/CA/NCI NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- R01 CA105312/CA/NCI NIH HHS/United States
- CA91842/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical