Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism - PubMed (original) (raw)
Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism
Edmond Y W Chan et al. Mol Cell Biol. 2009 Jan.
Abstract
The yeast Atg1 serine/threonine protein kinase and its mammalian homologs ULK1 and ULK2 play critical roles during the activation of autophagy. Previous studies have demonstrated that the conserved C-terminal domain (CTD) of ULK1 controls the regulatory function and localization of the protein. Here, we explored the role of kinase activity and intramolecular interactions to further understand ULK function. We demonstrate that the dominant-negative activity of kinase-dead mutants requires a 7-residue motif within the CTD. Our data lead to a model in which the functions of ULK1 and ULK2 are controlled by autophosphorylation and conformational changes involving exposure of the CTD. Additional mapping indicates that the CTD contains other distinct regions that direct membrane association and interaction with the putative human homologue of Atg13, which we have here characterized. Atg13 is required for autophagy and Atg9 trafficking during autophagy. However, Atg13 does not bind the 7-residue dominant-negative motif in the CTD of ULK proteins nor is the inhibitory activity of the CTDs rescued by Atg13 ectopic expression, suggesting that in mammalian cells, the CTD may interact with additional autophagy proteins.
Figures
FIG. 1.
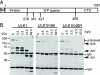
In vitro kinase activities of ULK1 mutants. (A and B) HEK293A cells were transfected with Myc-tagged ULK1 constructs, lysed, and used for immunoprecipitation reactions. Immunoprecipitates were incubated in an in vitro kinase reaction mixture containing [32P]ATP and the generic substrate MBP. Reaction products were resolved on SDS-PAGE gels (top). Anti-Myc immunoblotting was performed as a control to quantify the amount of ULK1 protein precipitated. Phosphorimager analysis detected levels of ULK1 autophosphorylation (P32-auto) and MBP phosphorylation (P32-MBP). Specific activities (in relative units) are expressed as the amount of phosphorylation normalized to the amount of Myc-tagged ULK1 per reaction (corrected for background activities detected in a parallel control reaction performed with untransfected cells [Untr] [not shown in panel B]). Each bar represents results from three independent samples ± standard deviations, and each experiment was reproduced a minimum of two times.
FIG. 2.
Inhibition of GFP-LC3 lipidation by ULK1-K46I. (A and B) 293/GFP-LC3 cells were transfected with a control plasmid expressing luciferase (pLUC) or the indicated Myc-tagged ULK1 constructs. Twenty-four hours after transfection, cells were either left alone or starved for 2 h in EBSS containing leupeptin and lysed for SDS-PAGE analysis of GFP-LC3 lipidation (conversion of GFP-LC3-I to GFP-LC3-II) by use of anti-LC3 antibodies. The membrane was also probed with anti-Myc and -β-tubulin (βTub) antibodies. GFP-LC3 lipidation is quantified as follows: GFP-LC3-II/(GFP-LC3-I + GFP-LC3-II). Each bar represents results from three independent samples ± standard deviations. (A) *, P < 0.05; NS, P = 0.20 (in pairwise comparisons with wild-type ULK1). (B) *, P < 0.05; NS, P = 0.18 (in pairwise comparisons with full-length ULK1-K46I). (C) Alignments show C-terminal amino acid residues from CTDs of ULK1 and ULK2. Positions of stop codons used to generate various C-terminal truncations are indicated at the top. ULK1(1-1001) is missing its C-terminal 50 residues; ΔCTD is a deletion of the entire CTD and corresponds to amino acids 1 to 828 of ULK1. The EGL-STOP mutant is further studied in Fig. 6F.
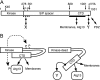
FIG. 3.
Differential sensitivities of ULK1 kinase mutants to limited proteolysis. (A) Schematic showing the domain structure of ULK1, including the N-terminal Myc tag (M), the kinase domain, the serine- and proline-rich spacer domain (S/P spacer), and the conserved CTD. Positions of stop codons used by Chan et al. (3) to generate various deletion mutants are indicated at the bottom. (B) ULK1 constructs transfected into 293A cells were immunoprecipitated and then incubated with the indicated concentrations of chymotrypsin for 15 min on ice. Reaction products were resolved on SDS-PAGE gels and detected by anti-Myc immunoblotting. Molecular mass markers are indicated on the left. The relative mobilities of the uncleaved proteins, previously described ULK1 truncation mutants, and the heavy and light immunoglobulin G (IgG) chains are shown on the right.
FIG. 4.
Autophagy regulatory roles of wild-type and kinase-dead ULK2. (A) In vitro autophosphorylation and MBP kinase activities for ULK2 constructs were measured as described in the legend for Fig. 1. (B, D, E, and F) Modulation of GFP-LC3 lipidation by ULK constructs was detected as described in the legend for Fig. 2. (B) *, P < 0.05; NS, P = 0.94 (in pairwise comparisons with pLUC-transfected, starved cells). (D) *, P < 0.03 in pairwise comparisons with pLUC-transfected cells at the same time point. (E) *, P < 0.06 in pairwise comparisons with pLUC-transfected, starved cells. (F) *, P < 0.02; NS, P = 0.42 (in pairwise comparisons with pLUC-transfected, starved cells). (C) In 293/GFP-LC3 cells starved in EBSS-leupeptin for 2 h, Myc-tagged ULK2 could be observed on multiple cytoplasmic structures, a portion of which colocalized with GFP-LC3, as indicated by arrows and the boxed inset. Bar = 10 μm. (G) Myc-tagged ULK1-K46I or ULK2-K39I inhibited the formation of cytoplasmic GFP-LC3-labeled autophagosomes in starved 293/GFP-LC3 cells. Bar = 10 μm. (H) Limited proteolysis of wild-type ULK2 and ULK2-K39I, assayed as described in the legend for Fig. 3. Untr, untransfected; P32-auto, ULK1 autophosphorylation; P32-MBP, MBP phosphorylation; βTub, β-tubulin; IgG, immunoglobulin G.
FIG. 5.
CTDs of ULK1 and ULK2 inhibit autophagy. (A) Control (pLUC) and expression constructs for Myc-tagged ULK1 and ULK2 CTDs were transfected into 293/GFP-LC3 cells. Inhibition of starvation-induced GFP-LC3 lipidation was measured as described in the legend for Fig. 2. **, P < 0.006 in pairwise comparisons with pLUC-transfected, starved cells. (B) 293/GFP-LC3 cells transfected as described for panel A were labeled overnight with [14C]valine. Cells were then either left untreated or starved in EBSS for 2 h, and autophagic degradation of long-lived proteins (prot deg) was analyzed. Cell samples transfected in parallel were used as a control to detect overexpressed constructs. Each bar represents the average from three independent samples ± the standard deviation. (C) HEK293A cells were transfected for 24 h with control pLUC plasmid, a Myc-tagged ULK1(1-351) deletion mutant as an internal control, or Myc-tagged ULK1 CTD. Cells were then left untreated or starved in EBSS-leupeptin for 2 h and then lysed for SDS-PAGE analysis of LC3 lipidation. The ratio of LC3-II/LC3-I measured for each sample is indicated at the bottom. (D) Myc-tagged ULK1 CTD constructs with C-terminal truncations were transfected into 293/GFP-LC3 cells, and GFP-LC3 lipidation was measured as described in the legend for Fig. 2. *, _P_ < 0.05; NS, _P_ > 0.4 (in pairwise comparisons with full-length-CTD-transfected, starved cells). A schematic of the truncations is shown in Fig. 2. βTub, β-tubulin.
FIG. 6.
Membrane targeting signal within the CTDs of ULK1 and ULK2. (A) 293/GFP-LC3 cells were transfected with Myc-ULK1 and starved in EBSS-leupeptin for 2 h. Cell homogenates were centrifuged at 100,000 × g to isolate membrane (Memb) and supernatant (Sup) fractions. Aliquots of the supernatant (representing 2% of the cell sample) and membrane (representing 10% of the cell sample) fractions were analyzed by SDS-PAGE. The top half of the blot was probed with anti-Myc and the bottom with anti-LC3. Where indicated, the membrane fraction was washed with HB containing 0.15 M or 0.5 M KCl. (B and C) Myc-tagged ULK1 and -2 constructs were transfected into cells and analyzed for membrane association as described above. (D) After transfection with Myc-tagged ULK1 CTD, cells were left untreated or starved in EBSS-leupeptin for 2 h. Where indicated, the membrane pellet was washed in HB containing 0.5 M KCl and analyzed as described above. (E) 293/GFP-LC3 cells were transfected with Myc-ULK1 CTD for 24 h and then starved in EBSS-leupeptin for 2 h before fixation and immunostaining with anti-Myc monoclonal antibody. In a Z section close to the substratum (Z = 0), ULK1 CTD strongly inhibited GFP-LC3 punctum formation. In the Z + 1 section, Myc-ULK1 CTD could be detected colocalizing with GFP-LC3-positive structures (inset). Bar = 10 μm. (F) Membrane associations of ULK1 CTD (Full CTD) and various C-terminal deletion constructs in starved 293/GFP-LC3 cells. See the schematic in Fig. 2 for the positions of inserted stop codons. The expression of ULK1 CTD was detected with anti-Myc, and GFP-LC3 was detected with anti-LC3. (G) Membrane associations of ULK1 CTD and two N-terminal deletion constructs, Myc-LHS (845-1051) and Myc-LKG (864-1051), in starved 293/GFP-LC3 cells. (H) 293/GFP-LC3 cells were transfected with full-length Myc-ULK1 or -ULK2 and then left untreated or starved in EBSS-leupeptin for 2 h before isolation of supernatant and membrane fractions. Expressed proteins were detected as described above. (I) Membrane association of transfected Myc-ULK1 in starved 293/GFP-LC3 cells. Membrane pellets were analyzed before washes (none) or following extraction in HB (0) or HB supplemented with 1.0% TX-100. (J) Membrane association of endogenous ULK1 was analyzed in untransfected, starved 293/GFP-LC3 cells following the fractionation and wash procedures described for panel I. WT, wild type; Untrans, untransfected.
FIG. 7.
Molecular complexes of ULK1 and ULK2 detected using native PAGE. HEK293A cells were transfected with ULK1 and ULK2 and starved for 2 h in EBSS where indicated (A) or ULK1 constructs (B) before lysis in native gel sample buffer. Complexes were resolved on native PAGE gels and then detected by immunoblotting with anti-Myc antibody (top). Positions of native-gel molecular mass markers are indicated on the left. As a control, aliquots of lysates were resolved by conventional SDS-PAGE to detect overexpressed proteins (anti-Myc) and total protein (β-tubulin [βTub]) (bottom). WT, wild type.
FIG. 8.
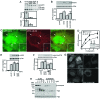
A putative human Atg13 homologue is required for autophagy. (A) 293/GFP-LC3 cells were transfected with control siRNA (Ctrl) or siRNA targeting ULK1 or a SMARTpool targeting KIAA0652 (Atg13) for 72 h. Cells were then left unstarved or starved in EBSS-leupeptin for 2 h before fixation and morphological analysis. The quantification represents the total GFP-LC3 spot intensity per cell (arbitrary units), and each bar represents the average of 60 image fields (from six independent cell samples) ± the standard deviation. **, P < 0.0007 in pairwise comparisons with Ctrl knockdown starved cells. (B) 293/GFP-LC3 cells were transfected for 72 h with control siRNA, the Atg13 SMARTpool, or individual duplexes (#1 or #2) specific for Atg13. Cells were left unstarved or starved as described for panel A and then lysed for immunoblot analysis of GFP-LC3 lipidation, as described in the legend for Fig. 2. *, P < 0.02 in pairwise comparisons with siRNA Ctrl starved cells. (C) HEK293A cells were transfected with control siRNA or Atg13 duplex 2 for 72 h. Cells were then left unstarved (Unst) or starved (st) for 2 h in EBSS (with or without leupeptin [Leu]) before lysis for immunoblot analysis of endogenous LC3 lipidation. The quantified ratio of LC3-II/LC3-I is shown below each sample. βTub, β-tubulin. (D) 293/GFP-LC3 cells were transfected for 72 h with control siRNA (siCtrl) or siRNAs targeting ULK1 (siULK1) or Atg13 (duplex 2) (siAtg13), starved in EBSS-leupeptin for 2 h, and then fixed and immunostained to detect endogenous mAtg9 localization (red). After starvation in siCtrl-treated cells, mAtg9 becomes redistributed predominantly to a diffuse cytoplasmic pool. After knockdown of Atg13, mAtg9 redistribution is blocked and mAtg9-positive vesicles remain in juxtanuclear clusters (arrows) to an extent similar to that after knockdown of ULK1. Inhibition of starvation-induced GFP-LC3 autophagosome formation after knockdown of ULK1 and Atg13 is shown as a control. Bar = 10 μm.
FIG. 9.
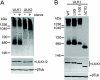
Human Atg13 is membrane associated. (A) 293/GFP-LC3 cells expressing Atg13-FLAG were starved for 2 h in EBSS-leupeptin, fixed, and then immunostained using anti-FLAG antibody. Atg13-FLAG can be found both in a diffuse cytosolic pool and localized to punctate structures that do not colocalize with GFP-LC3. Bar = 10 μm. (B) Membrane association of Atg13-FLAG was analyzed as described in the legend for Fig. 6. (C) HEK293A cells were transfected with control or Atg13 duplex 2 (Dup#2) for 72 h. Cell lysates were then immunoblotted with a polyclonal antibody for endogenous Atg13 or actin as a loading control. (D) Membrane fractions analyzed as described for Fig. 6J from untransfected 293/GFP-LC3 cells were probed for endogenous Atg13. Sup, supernatant; Memb, membrane fraction; Ctrl, control.
FIG. 10.
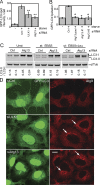
Binding of Atg13 to the CTD is required for phosphorylation by ULK1 and ULK2. (A, B, and C) HEK293A cells were transfected with Atg13-FLAG alone (−) or in combination (+) with Myc-ULK1, Myc-ULK2, Myc-ULK1-K46I, or Myc-ULK2-K39I, as indicated. An aliquot of cell lysate was analyzed by SDS-PAGE and immunoblotting with Myc (top) and FLAG (bottom) antibodies. Lysates of doubly transfected cells were incubated with anti-FLAG beads to coimmunoprecipitate Atg13 and ULK proteins. (D) Myc-ULK2 and Myc-ULK2ΔCTD were coexpressed with FLAG-Atg13 in HEK293A cells. As a control, Atg13-FLAG was expressed alone (−). Cell lysates were immunoprecipitated with anti-Myc or anti-FLAG antibody and then immunoblotted as described above. (E) Coimmunoprecipitation of the Myc-tagged CTDs of ULK1 and ULK2 with Atg13-FLAG. Lysates and immunoprecipitates were analyzed as described above. (F) Coimmunoprecipitation of Myc-ULK1CTD(829-1001) with Atg13-FLAG. Lysates and immunoprecipitates were analyzed as described above. The asterisks in panels D, E, and F indicate nonspecific bands arising from the antibody used for immunoprecipitation, as demonstrated by the antibody-plus-beads-alone control (Ab ctrl). (G) 293/GFP-LC3 cells were cotransfected with Myc-ULK1 CTD or Myc-ULK2CTD with or without Atg13-FLAG for 24 h and then either left unstarved or starved in EBSS-leupeptin for 2 h before lysis for analysis of GFP-LC3 lipidation, as described in the legend for Fig. 2. Protein expression controls (top) using anti-FLAG and anti-Myc antibodies are shown. (H) Immunoprecipitated Atg13-FLAG protein was used as a substrate for immunoprecipitated Myc-ULK proteins in a mixed-bead in vitro kinase reaction. Phosphorylation of Atg13-FLAG is detected as a decrease in mobility. A control reaction without the addition of ULK proteins (−) shows no mobility shift. (I) HEK293A cells were either left untransfected (−) or transfected with Myc-ULK1 or Myc-ULK2 before cell lysates were immunoblotted to detect hypershifting on endogenous Atg13. Lys, lysates; IP, immunoprecipitate.
FIG. 11.
Conformational changes of ULK proteins modulated by autophosphorylation status. (A) Domain structure of ULK1 showing the N-terminal kinase domain and the critical lysine 46 residue (K46) targeted in our mutant constructs. Our data are consistent with the existence of multiple, but so far unidentified, autophosphorylation (P) sites within residues 278 to 351 of the serine- and proline-rich spacer domain (S/P spacer). Our previous findings indicate a region spanning residues 278 to 351 that is critical for inhibition of autophagy, presumably via interaction with an unknown protein (X). Our previous data also indicated that the last three residues of ULK1 (VYA) are critical for regulatory function, possibly by binding a PDZ domain-containing protein. Our findings here indicate multiple functions directed by sequences within the conserved CTD. A region between residues 829 and 1001 contains signals for binding to membranes and interaction with human Atg13. Additional sequences between residues 1038 and 1044 (Fig. 2) contain a conserved motif (IERRLSA, black diamond) that is required for dominant inhibitory activity by interacting with another unknown factor (Y). (B) Proposed model showing phosphorylation-dependent conformational changes. In normal resting cells, ULK proteins are autophosphorylated, and these modifications help maintain a closed conformation that (i) brings Atg13 in closer proximity to the kinase domain for phosphorylation and (ii) keeps the dominant-negative motif hidden or inaccessible. Full ablation of kinase activity results in a low degree of autophosphorylation and a more open molecular conformation that exposes the dominant inhibitory CTD motif, allowing it to bind the unknown factor (Y) that is critical for autophagy.
Similar articles
- ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery.
Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. Jung CH, et al. Mol Biol Cell. 2009 Apr;20(7):1992-2003. doi: 10.1091/mbc.e08-12-1249. Epub 2009 Feb 18. Mol Biol Cell. 2009. PMID: 19225151 Free PMC article. - Structure of the Human Atg13-Atg101 HORMA Heterodimer: an Interaction Hub within the ULK1 Complex.
Qi S, Kim DJ, Stjepanovic G, Hurley JH. Qi S, et al. Structure. 2015 Oct 6;23(10):1848-1857. doi: 10.1016/j.str.2015.07.011. Epub 2015 Aug 20. Structure. 2015. PMID: 26299944 Free PMC article. - A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy.
Mercer CA, Kaliappan A, Dennis PB. Mercer CA, et al. Autophagy. 2009 Jul;5(5):649-62. doi: 10.4161/auto.5.5.8249. Epub 2009 Jul 20. Autophagy. 2009. PMID: 19287211 - ATG13: just a companion, or an executor of the autophagic program?
Alers S, Wesselborg S, Stork B. Alers S, et al. Autophagy. 2014 Jun;10(6):944-56. doi: 10.4161/auto.28987. Autophagy. 2014. PMID: 24879146 Free PMC article. Review. - Canonical and noncanonical functions of ULK/Atg1.
Wang B, Kundu M. Wang B, et al. Curr Opin Cell Biol. 2017 Apr;45:47-54. doi: 10.1016/j.ceb.2017.02.011. Epub 2017 Mar 11. Curr Opin Cell Biol. 2017. PMID: 28292700 Free PMC article. Review.
Cited by
- AAV-mediated inhibition of ULK1 promotes axonal regeneration in the central nervous system in vitro and in vivo.
Ribas VT, Vahsen BF, Tatenhorst L, Estrada V, Dambeck V, Almeida RA, Bähr M, Michel U, Koch JC, Müller HW, Lingor P. Ribas VT, et al. Cell Death Dis. 2021 Feb 26;12(2):213. doi: 10.1038/s41419-021-03503-3. Cell Death Dis. 2021. PMID: 33637688 Free PMC article. - Proteotoxic stress induces phosphorylation of p62/SQSTM1 by ULK1 to regulate selective autophagic clearance of protein aggregates.
Lim J, Lachenmayer ML, Wu S, Liu W, Kundu M, Wang R, Komatsu M, Oh YJ, Zhao Y, Yue Z. Lim J, et al. PLoS Genet. 2015 Feb 27;11(2):e1004987. doi: 10.1371/journal.pgen.1004987. eCollection 2015. PLoS Genet. 2015. PMID: 25723488 Free PMC article. - The ULK1 complex mediates MTORC1 signaling to the autophagy initiation machinery via binding and phosphorylating ATG14.
Park JM, Jung CH, Seo M, Otto NM, Grunwald D, Kim KH, Moriarity B, Kim YM, Starker C, Nho RS, Voytas D, Kim DH. Park JM, et al. Autophagy. 2016;12(3):547-64. doi: 10.1080/15548627.2016.1140293. Autophagy. 2016. PMID: 27046250 Free PMC article. - ULK3-dependent activation of GLI1 promotes DNMT3A expression upon autophagy induction.
González-Rodríguez P, Cheray M, Keane L, Engskog-Vlachos P, Joseph B. González-Rodríguez P, et al. Autophagy. 2022 Dec;18(12):2769-2780. doi: 10.1080/15548627.2022.2039993. Epub 2022 Feb 28. Autophagy. 2022. PMID: 35226587 Free PMC article. - Potential ceRNA networks involved in autophagy suppression of pancreatic cancer caused by chloroquine diphosphate: A study based on differentially‑expressed circRNAs, lncRNAs, miRNAs and mRNAs.
Wei DM, Jiang MT, Lin P, Yang H, Dang YW, Yu Q, Liao DY, Luo DZ, Chen G. Wei DM, et al. Int J Oncol. 2019 Feb;54(2):600-626. doi: 10.3892/ijo.2018.4660. Epub 2018 Dec 10. Int J Oncol. 2019. PMID: 30570107 Free PMC article.
References
- Chan, E. Y., S. Kir, and S. A. Tooze. 2007. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J. Biol. Chem. 28225464-25474. - PubMed
- Cheong, H., and D. J. Klionsky. 2008. Dual role of Atg1 in regulation of autophagy-specific PAS assembly in Saccharomyces cerevisiae. Autophagy 4724-726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases