Evaluation of Dimebon in cellular model of Huntington's disease - PubMed (original) (raw)
Evaluation of Dimebon in cellular model of Huntington's disease
Jun Wu et al. Mol Neurodegener. 2008.
Abstract
Background: Dimebon is an antihistamine compound with a long history of clinical use in Russia. Recently, Dimebon has been proposed to be useful for treating neurodegenerative disorders. It has demonstrated efficacy in phase II Alzheimer's disease (AD) and Huntington's disease (HD) clinical trials. The mechanisms responsible for the beneficial actions of Dimebon in AD and HD remain unclear. It has been suggested that Dimebon may act by blocking NMDA receptors or voltage-gated Ca2+ channels and by preventing mitochondrial permeability pore transition.
Results: We evaluated the effects of Dimebon in experiments with primary striatal neuronal cultures (MSN) from wild type (WT) mice and YAC128 HD transgenic mice. We found that Dimebon acts as an inhibitor of NMDA receptors (IC50 = 10 muM) and voltage-gated calcium channels (IC50 = 50 muM) in WT and YAC128 MSN. We further found that application of 50 muM Dimebon stabilized glutamate-induced Ca2+ signals in YAC128 MSN and protected cultured YAC128 MSN from glutamate-induced apoptosis. Lower concentrations of Dimebon (5 muM and 10 muM) did not stabilize glutamate-induced Ca2+ signals and did not exert neuroprotective effects in experiments with YAC128 MSN. Evaluation of Dimebon against a set of biochemical targets indicated that Dimebon inhibits alpha-Adrenergic receptors (alpha1A, alpha1B, alpha1D, and alpha2A), Histamine H1 and H2 receptors and Serotonin 5-HT2c, 5-HT5A, 5-HT6 receptors with high affinity. Dimebon also had significant effect on a number of additional receptors.
Conclusion: Our results suggest that Ca2+ and mitochondria stabilizing effects may, in part, be responsible for beneficial clinical effects of Dimebon. However, the high concentrations of Dimebon required to achieve Ca2+ stabilizing and neuroprotective effects in our in vitro studies (50 muM) indicate that properties of Dimebon as cognitive enhancer are most likely due to potent inhibition of H1 histamine receptors. It is also possible that Dimebon acts on novel high affinity targets not present in cultured MSN preparation. Unbiased evaluation of Dimebon against a set of biochemical targets indicated that Dimebon efficiently inhibited a number of additional receptors. Potential interactions with these receptors need to be considered in interpretation of results obtained with Dimebon in clinical trials.
Figures
Figure 1
Chemical structure of Dimebon. Chemical structure of 2,3,4,5-Tetrahydro-2,8-dimethyl-5-(2-(6-methyl-3-pyridyl)ethyl)-1H-pyrido(4,3-b)indole (CAS 3613-73-8)
Figure 2
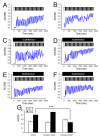
Effects of Dimebon on glutamate-induced Ca2+signals. (A-B), Repetitive application of 20 μM glutamate induces Ca2+ signals in MSN from the WT (A) and YAC128 (B) mice. (C-D), The same experiment as in (A) and (B) was performed in the presence of 10 μM Dimebon with WT (C) and YAC128 (D) MSN. (E-F), The same experiment as in (A) and (B) was performed in the presence of 50 μM Dimebon with WT (E) and YAC128 (F) MSN. The traces shown on panels (A-F) are average traces from all MSN for each experimental group. (G) The average increase of basal Ca2+ level (mean ± SE, n is the number of MSN analyzed) after 20 pulses of glutamate are shown for WT MSN (n = 16), YAC128 MSN (n = 21), WT MSN in the presence of 10 μM Dimebon (n = 44), YAC128 MSN in the presence of 10 μM Dimebon (n = 41), WT MSN in the presence of 50 μM Dimebon (n = 17) and YAC128 MSN in the presence of 50 μM Dimebon (n = 44).
Figure 3
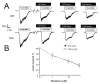
Effects of Dimebon on NMDA-evoked currents. (A) Representative traces of NMDA-evoked current recorded from DIV9-10 WT and YAC128 MSN. The white bar represents application of 100 μM NMDA in the presence of 50 μM glycine and black bar represents application of 1 μM, 10 μM and 50 μM Dimebon as indicated. (B) Dose-dependence of Dimebon block. The peak NMDA-induced currents were normalized to the peak NMDA-induced currents in the same cell in the absence of Dimebon. The normalized data at each Dimebon concentration were averaged from several experiments and shown as mean ± SE (n = 8 for WT and n = 9 for YAC128 MSN). The IC50 = 10 μM for WT and YAC128 MSN.
Figure 4
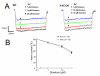
Effects of Dimebon on voltage-gated calcium currents. (A) Representative traces of voltage-gated Ca2+ currents evoked by membrane depolarization from -80 mV holding potential to 0 mV in DIV9 WT and YAC128 MSN. The current waveforms recorded in the same cell in the absence of Dimebon (open circle), and in the presence of 1 μM (filled circle), 10 μM (open triangle) and 50 μM (filled diamond) of Dimebon are shown. (B) Dose-dependence of Dimebon block of voltage-gated Ca2+ channels. The peak voltage-gated Ca2+ currents were normalized to the peak voltage-gated Ca2+ currents recorded in the same cell in the absence of Dimebon. The normalized data at each Dimebon concentration were averaged from several experiments and shown as mean ± SE (n = 14 for WT and n = 15 forYAC128 MSN). The IC50 = 50 μM for WT and YAC128 MSN.
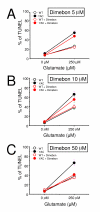
Figure 5
Evaluation of Dimebon in in vitro HD assay. Glutamate-induced apoptosis of WT and YAC128 MSN treated with Dimebon at different concentrations. WT and YAC128 MSN at 14 DIV were exposed to 250 μM glutamate for 7 h, fixed, permeabilized and analyzed by TUNEL staining and PI counterstaining. The Dimebon was added 30 min before the application of glutamate. The fraction of TUNEL-positive is plotted against glutamate concentration for WT (open circle) and YAC128 (YAC, filled circles) mice. The data are shown as mean ± SE (n = 6–8 microscopic fields, 100–300 MSN per field). The results in the absence (black symbols) and presence (red symbols) obtained in the presence of 5 μM of Dimebon (A) 10 μM Dimebon (B) and 50 μM Dimebon (C) are compared.
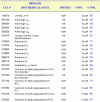
Figure 6
Significant biochemical targets of Dimebon. Significant biochemical targets of Dimebon are shown. The Cat numbers refer to the MDS Pharma Services assay specification. The receptors and species (human or rat) are listed. Dimebon was tested at 10 μM concentration. The % inhibition is shown for each receptor. Significant targets are defined as % inhibition > 50%.
Similar articles
- Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease.
Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinás R, Kristal BS, Hayden MR, Bezprozvanny I. Tang TS, et al. Proc Natl Acad Sci U S A. 2005 Feb 15;102(7):2602-7. doi: 10.1073/pnas.0409402102. Epub 2005 Feb 3. Proc Natl Acad Sci U S A. 2005. PMID: 15695335 Free PMC article. - From anti-allergic to anti-Alzheimer's: Molecular pharmacology of Dimebon.
Okun I, Tkachenko SE, Khvat A, Mitkin O, Kazey V, Ivachtchenko AV. Okun I, et al. Curr Alzheimer Res. 2010 Mar;7(2):97-112. doi: 10.2174/156720510790691100. Curr Alzheimer Res. 2010. PMID: 19939222 - The rise and fall of Dimebon.
Bezprozvanny I. Bezprozvanny I. Drug News Perspect. 2010 Oct;23(8):518-23. doi: 10.1358/dnp.2010.23.8.1500435. Drug News Perspect. 2010. PMID: 21031168 Free PMC article. - New Therapeutic Property of Dimebon as a Neuroprotective Agent.
Ustyugov A, Shevtsova E, Ashraf GM, Tarasov VV, Bachurin SO, Aliev G. Ustyugov A, et al. Curr Med Chem. 2018;25(39):5315-5326. doi: 10.2174/0929867323666160804122746. Curr Med Chem. 2018. PMID: 27494393 Review. - Novel Sites of Neuroprotective Action of Dimebon (Latrepirdine).
Ustyugov A, Shevtsova E, Bachurin S. Ustyugov A, et al. Mol Neurobiol. 2015 Oct;52(2):970-8. doi: 10.1007/s12035-015-9249-4. Epub 2015 Jun 30. Mol Neurobiol. 2015. PMID: 26123670 Review.
Cited by
- Preclinical study of dimebon on β-amyloid-mediated neuropathology in Alzheimer's disease.
Wang J, Ferruzzi MG, Varghese M, Qian X, Cheng A, Xie M, Zhao W, Ho L, Pasinetti GM. Wang J, et al. Mol Neurodegener. 2011 Jan 19;6(1):7. doi: 10.1186/1750-1326-6-7. Mol Neurodegener. 2011. PMID: 21247479 Free PMC article. - Enhanced Store-Operated Calcium Entry Leads to Striatal Synaptic Loss in a Huntington's Disease Mouse Model.
Wu J, Ryskamp DA, Liang X, Egorova P, Zakharova O, Hung G, Bezprozvanny I. Wu J, et al. J Neurosci. 2016 Jan 6;36(1):125-41. doi: 10.1523/JNEUROSCI.1038-15.2016. J Neurosci. 2016. PMID: 26740655 Free PMC article. - Mitochondrial dysfunction--a pharmacological target in Alzheimer's disease.
Eckert GP, Renner K, Eckert SH, Eckmann J, Hagl S, Abdel-Kader RM, Kurz C, Leuner K, Muller WE. Eckert GP, et al. Mol Neurobiol. 2012 Aug;46(1):136-50. doi: 10.1007/s12035-012-8271-z. Epub 2012 May 3. Mol Neurobiol. 2012. PMID: 22552779 Review. - Calcium signaling and neurodegenerative diseases.
Bezprozvanny I. Bezprozvanny I. Trends Mol Med. 2009 Mar;15(3):89-100. doi: 10.1016/j.molmed.2009.01.001. Epub 2009 Feb 21. Trends Mol Med. 2009. PMID: 19230774 Free PMC article. - Corticostriatal circuit dysfunction in Huntington's disease: intersection of glutamate, dopamine and calcium.
Miller BR, Bezprozvanny I. Miller BR, et al. Future Neurol. 2010 Sep;5(5):735-756. doi: 10.2217/fnl.10.41. Future Neurol. 2010. PMID: 21977007 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous