JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut - PubMed (original) (raw)
JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut
Benoît Biteau et al. Cell Stem Cell. 2008.
Abstract
Metazoans employ cytoprotective and regenerative strategies to maintain tissue homeostasis. Understanding the coordination of these strategies is critical to developing accurate models for aging and associated diseases. Here we show that cytoprotective Jun N-terminal kinase (JNK) signaling influences regeneration in the Drosophila gut by directing proliferation of intestinal stem cells (ISCs). Interestingly, this function of JNK contributes to the loss of tissue homeostasis in old and stressed intestines by promoting the accumulation of misdifferentiated ISC daughter cells. Ectopic Delta/Notch signaling in these cells causes their abnormal differentiation but also limits JNK-induced proliferation. Protective JNK signaling and control of cell proliferation and differentiation by Delta/Notch signaling thus have to be carefully balanced to ensure tissue homeostasis. Our findings suggest that this balance is lost in old animals, increasing the potential for neoplastic transformation.
Figures
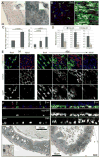
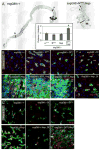
Figure 1. Tissue degeneration in the aging Drosophila gut
A. ISCs and EBs in young (3 days) and old (40 days) guts labeled by X-Gal staining (genotype: y,w;P{lacW}esgk00606/CyO). Inserts show overview of the depicted guts (A: anterior; P: posterior; endogenous β-Galactosidase Figure S1). B. Confocal images of aging guts from flies expressing GFP in ISCs and EBs (genotype: w1118; esgGal4, UASGFP). ISCs and EBs are labeled by GFP expression (esgGal4>UASGFP, green) in young flies. Cell boundaries are labeled by immunostaining against Armadillo (membrane red), EE cells are labeled by nuclear pros staining (nuclear red). DNA detected by Hoechst staining (blue). Additional images are shown in Supplementary Figure S2. C. Age-related changes in the intestinal epithelium quantified by measuring the averages of cell number and size in esg+ cell clusters. Relatively young guts (3, 10 and 15 days) were scored to allow for accurate quantification of the observed parameters. Widespread disorganization (compare with B) prevents accurate identification of individual clusters of cells in older guts. p-values were determined using Student’s t-test. D. Reduction of trypsin expression in the aging gut. Expression of three different trypsin isoforms was measured by real-time RT-PCR in cDNA prepared from dissected guts from young and old flies. Expression is normalized to the expression of the rp49 gene. p-values were calculated using Student’s t-test: * p<0.05; **p<0.01. **E.** Confocal images of guts from young and old flies expressing GFP under the control of esgGal4. Fer1HCH, detected by immunostaining (red), is expressed specifically in ECs in young flies. **F.** Cross-sections of guts from old and young esgGal4>UASGFP flies illustrating the loss of the monolayered architecture of the intestinal epithelium in older animals. ISCs and EBs are GFP positive (green), EE cells are labeled by nuclear pros staining (red) and DNA detected by Hoechst staining (blue). G. Ultrastructural analysis of cross-sections of young and old guts using Transmission Electron Microscopy. Blue asterisks indicate enterocytes, red asterisks mark ISCs or cells accumulating in the basal part of the epithelium. A representative ISC in young intestines is outlined in the insert of the left panel. Additional and higher magnification images are presented in Supplementary Figure S5.
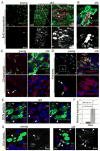
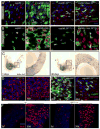
Figure 2. Increased proliferation and deregulation of Notch signaling in old guts
A, B. Increased rate of ISC proliferation in old intestines. Young and old flies were fed BrdU for 4 days. BrdU incorporation can be detected in both small cells (ISCs, EBs; open arrowheads), that divided while BrdU was being provided, and in larger nuclei characterizing ECs that underwent differentiation (including endoreplication; closed arrowheads). Panel B shows an isolated cluster of aberrant esg+ cells in the gut of 60-day-old flies. BrdU labeling of adjacent small and larger nuclei indicate lineage relationship between the labeled cells. Large esg+ cells that were formed before exposure to BrdU, are not labeled with BrdU, highlighting the continued expression of the esg>GFP marker in ISC progeny of old flies. C. Distribution of Delta protein is aberrant in old guts. Delta is labeled by immunostaining (red), ISCs and EBs are identified by GFP expression (esgGal4>GFP, green) and DNA by Hoechst staining (blue). D. Activity of the Notch signaling pathway monitored using the Su(H)-GBE-lacZ reporter line, which is specifically expressed in EBs in young guts. β-galactosidase was detected by immunostaining (red). GFP (green) identifies ISCs and EBs. E. Co-expression of Delta and Notch signaling reporter in the abnormal progeny of ISCs in old flies. Confocal images of posterior midgut from 35-day-old Su(H)-GBE-lacZ/+ flies, Delta is detected by immunostaining (red), β-galactosidase expression identifies cell with elevated Notch signaling (green). Note the presence of cells with large nuclei (blue) that are both Dl- and β-galactosidase-positive (close arrowheads). Cells retaining ISC identity (small nuclei, Dl-positive and β-galactosidase-negative) are also indicated (open arrowheads). F. Expression of Dl was measured by real-time RT-PCR in cDNA prepared from dissected guts from young and old flies. Expression is normalized to the expression of the rp49 gene. p-values were calculated using Student’s t-test: * p<0.05. G. Positively marked clones (lacZ+) were generated in the gut of young (left) and old (right) flies as described in (Harrison and Perrimon, 1993). Note the presence of a single Dl+ cell in clone from young animal and multiple Dl+ cells in clone generated in the gut of older animals.
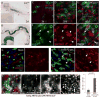
Figure 3. JNK activity increases with age in the gut and is required for stress tolerance of ECs
A, B Expression of lacZ in the pucE69 line was monitored to detect JNK activation in young (2 days, A) and old (50 days, B). Inserts show higher magnification of the boxed area (A: anterior, P: posterior). C-F Nuclear β-galactosidase accumulates in aging _puc_E69 guts, but is not detected in ISCs (genotype esgGal4, UASGFP; pucE69/+). Projections of confocal Z-stacks. GFP identifies ISCs and EBs (green). β-galactosidase (red) and Arm and Pros (blue) expression detected by immunostaining. (C–E) posterior midguts at 2 days (C), 20 days (D), 60 days (E) of age. (F) Closeup of E. High levels of nuclear lacZ is detected in ECs (closed arrowhead points out one example) and EEs (red arrowhead), but not in ISCs (small GFP+ cells (open arrowhead)). Note that GFP-positive cells with larger nuclei also express lacZ (green arrowhead points out one example). G, H puc expression is induced by Paraquat exposure in young guts. 5-day-old animals exposed to 5mM Paraquat (PQ, H) or carrier (Mock, G) for 24 hours. Puc-lacZ expression is also observed in ISCs (arrowheads). I: bsk2 mutant clones marked by the absence of GFP (green; genotype is hsFlp; FRT40 ubi-GFP/FRT40 bsk2). Guts are from 15 day-old flies exposed to 5mM Paraquat for 12 hrs and guts were immunolabeled against the activated form of the Drosophila Caspase3 Drice (red; gift from Bruce Hay). Note elevated Driceact staining in bsk2 mutant ECs (GFP−, closed arrowheads). A Driceact positive wild-type EC is also indicated (open arrowheads). The proportion of bsk mutant cells in the whole tissue and among Driceact positive cells was calculated.
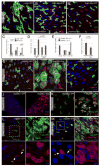
Figure 4. JNK-dependent degeneration of epithelial architecture in the intestine
A–D. Age-related changes in the intestinal epithelium are reduced in JNK loss-of-function conditions. 20-day-old males hemizygous for hep1 (B and B′ are independent samples of identical genotypes) are compared to wild-type controls (A) reared under identical conditions. The number of guts containing clusters of esg+ cells is compared between populations of hep1 hemizygotes and wild-type controls in C. Three classes of esg+ overgrowth are represented by panels A (“strong overgrowth”), B (“intermediate”), and B′ (“individual ISCs”). Averages and SEM of three independent double-blind quantifications are shown. P values from Student’s T test. Quantification in D is as described in Figure 1D. E, F. Similar reduction of age-associated expansion of esg+ population is seen in flies over-expressing dominant-negative Bsk (BskDN) in ISCs. The TARGET system was used to over-express BskDN under the control of esgGal4. Flies were aged for 15 days at 29°C. G–I. JNK activation is sufficient to induce phenotypes similar to the one observed in old flies. Posterior midguts of WT control flies (esgGal4, UASGFP;tubGal80ts) and flies with moderate activation of JNK (esgGal4, UASGFP/UASHep; tubGal80ts) after 3 days at 29°C. Green: GFP; red: arm, pros; blue: DNA. The phenotype observed in Hep over-expressing flies is reversed when dominant-negative JNK (BskDN) is co-overexpressed (I). J, K. JNK activation induces ISCs proliferation. Flies were fed BrdU for 2 days and then incubated at 29°C for 2 days. Over-expression of Hep in ISCs and EBs induces widespread BrdU incorporation in esg+ cell clusters, identifying stem cells that recently divided (arrowheads in K), as well as differentiating cells undergoing endoreplication. L, M. JNK activation induces perturbation in Delta distribution and in Notch signaling. Delta (red in L) and the Notch reporter Su(H)-GBE-lacZ (red in M) are expressed respectively in ISCs and EB in control flies. Moderate activation of JNK (esgGal4, UASGFP/UASHep; tubGal80ts), for 3–5 days, leads to the formation of cluster of esg+ cells expressing high level of delta and showing high Notch signaling activity. Green: GFP; red: delta in L and β-galactosidase in M; blue: DNA.
Figure 5. JNK and Delta/Notch signaling coordinate stem cell proliferation
A. Co expression of Hep and NotchRNAi in ISCs and EBs results in dramatic changes in gut morphology. Representative pictures of intestines from wild-type flies and flies expressing Hep and NotchRNAi under the control of the TARGET system (genotype: esgGal4, UASGFP, tub>Gal80ts/UASHep; UASNRNAi). The phenotype was quantified by measuring the ratio between the width of the posterior and anterior midguts. A: anterior, P: posterior; the two red lines indicate where the width of the gut was measured. * p-value <10−4, compared to the 3 other genotypes using Student’s t-test. B, C. Confocal images of the posterior midgut of flies expressing Hep in combination with Delta/Notch signaling gain- or loss-of-function. After 5 days of induction at 29°C, in flies expressing both Hep+NotchRNAi or Hep+DeltaRNAi, ISCs populate the entire intestinal epithelium, whereas Delta over-expression blocks Hep-induced changes in ISC number and morphology. (C) The effects of co-expression of DeltaRNAi or UASDelta on Hep-induced proliferation were confirmed by assessing BrdU incorporation in intestinal cells. Note that Delta over-expression inhibits JNK-induced proliferation of ISCs.
Figure 6. Reduction of delta gene dose prevents misdifferentiation, but not over-proliferation of ISCs in old flies and in response to JNK activation
A, B. Reduction of delta gene dose prevents JNK-induced misdifferentiation but not JNK-induced proliferation. (A) Over-expression of Hep in ISCs and EBs, using esgGal4, in a Dl05151/+ background does not result in expansion of the esg+ cell population. (B) The same flies were fed BrdU for 4 days. BrdU incorporation shows that Hep over-expression induces proliferation in Dl05151/+ background. Similar results were observed using another Dl loss of function allele (Dl7; data not shown). C. The enhancer-trap loss-of-function allele Dl05151 was used to identify Dl expressing cells in the posterior midgut from young and old flies, by X-gal staining. Right panels show higher magnification of boxed area; A: anterior, P: posterior. D, E. Reduction of delta gene dose (heterozygotes for Dl05151) is sufficient to prevent age-associated loss of epithelial architecture in the gut. Guts from 40-day-old Dl05151/+ (D) or esg>GFP; Dl05151/+ (E) flies do not show the age-induced deterioration of the intestinal epithelium observed in wild-type animals (compare D with Figure S2). green: GFP; red: armadillo and prospero; blue: DNA. Similar results were obtained with an independent Dl allele (Dl7, Figure S11). F. 3-day-old and 30-day-old Dl05151/+ flies and their isogenic control ry506 were fed BrdU for 4 days. Similar age-related increase in BrdU incorporation is observed in Dl heterozygotes and wild-type flies.
Figure 7. Model for the effects of JNK and Notch signaling in the aging and stressed intestine
JNK is required for the transcriptional induction of damage repair and stress response genes (such as thor and small heat shock proteins (Wang et al., 2005)), promoting cytoprotection of intestinal cells. At the same time, JNK activation induces proliferation of ISCs. When animals are exposed to high levels or chronic stress, this activity of JNK promotes the accumulation of Dl+ EBs that activate Notch signaling in neighboring cells, resulting in misdifferentiation and loss of tissue homeostasis. In Dl heterozygotes, ectopic Dl expression is reduced, so that differentiation can proceed normally. When Notch signaling is impaired more efficiently by DlRNAi or NRNAi, however, EB differentiation is inhibited, resulting in JNK-mediated over-proliferation of ISCs and loss of epithelial integrity.
Similar articles
- The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila.
Park JS, Kim YS, Yoo MA. Park JS, et al. Aging (Albany NY). 2009 May 21;1(7):637-51. doi: 10.18632/aging.100054. Aging (Albany NY). 2009. PMID: 20157545 Free PMC article. - Lifespan extension by preserving proliferative homeostasis in Drosophila.
Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Biteau B, et al. PLoS Genet. 2010 Oct 14;6(10):e1001159. doi: 10.1371/journal.pgen.1001159. PLoS Genet. 2010. PMID: 20976250 Free PMC article. - Control of Intestinal Cell Fate by Dynamic Mitotic Spindle Repositioning Influences Epithelial Homeostasis and Longevity.
Hu DJ, Jasper H. Hu DJ, et al. Cell Rep. 2019 Sep 10;28(11):2807-2823.e5. doi: 10.1016/j.celrep.2019.08.014. Cell Rep. 2019. PMID: 31509744 Free PMC article. - Intestinal stem cells in the adult Drosophila midgut.
Jiang H, Edgar BA. Jiang H, et al. Exp Cell Res. 2011 Nov 15;317(19):2780-8. doi: 10.1016/j.yexcr.2011.07.020. Epub 2011 Aug 11. Exp Cell Res. 2011. PMID: 21856297 Free PMC article. Review. - Drosophila model systems reveal intestinal stem cells as key players in aging.
Park JS, Sung MJ, Na HJ. Park JS, et al. Ann N Y Acad Sci. 2025 May;1547(1):88-99. doi: 10.1111/nyas.15351. Epub 2025 Apr 25. Ann N Y Acad Sci. 2025. PMID: 40276941 Review.
Cited by
- Requirement of ATR for maintenance of intestinal stem cells in aging Drosophila.
Park JS, Na HJ, Pyo JH, Jeon HJ, Kim YS, Yoo MA. Park JS, et al. Aging (Albany NY). 2015 May;7(5):307-18. doi: 10.18632/aging.100743. Aging (Albany NY). 2015. PMID: 26000719 Free PMC article. - Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila.
Rera M, Clark RI, Walker DW. Rera M, et al. Proc Natl Acad Sci U S A. 2012 Dec 26;109(52):21528-33. doi: 10.1073/pnas.1215849110. Epub 2012 Dec 12. Proc Natl Acad Sci U S A. 2012. PMID: 23236133 Free PMC article. - The midgut epithelium of mosquitoes adjusts cell proliferation and endoreplication to respond to physiological challenges.
Taracena-Agarwal ML, Hixson B, Nandakumar S, Girard-Mejia AP, Chen RY, Huot L, Padilla N, Buchon N. Taracena-Agarwal ML, et al. BMC Biol. 2024 Jan 29;22(1):22. doi: 10.1186/s12915-023-01769-x. BMC Biol. 2024. PMID: 38281940 Free PMC article. - The Drosophila midgut: a model for stem cell driven tissue regeneration.
Lucchetta EM, Ohlstein B. Lucchetta EM, et al. Wiley Interdiscip Rev Dev Biol. 2012 Sep-Oct;1(5):781-8. doi: 10.1002/wdev.51. Epub 2012 Apr 9. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23799573 Free PMC article. - The multi-tasking gut epithelium of insects.
Huang JH, Jing X, Douglas AE. Huang JH, et al. Insect Biochem Mol Biol. 2015 Dec;67:15-20. doi: 10.1016/j.ibmb.2015.05.004. Epub 2015 May 14. Insect Biochem Mol Biol. 2015. PMID: 25982023 Free PMC article. Review.
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. - PubMed
- Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. - PubMed
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous