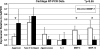Use of bone morphogenic protein-7 as a treatment for osteoarthritis - PubMed (original) (raw)
Use of bone morphogenic protein-7 as a treatment for osteoarthritis
Neil Badlani et al. Clin Orthop Relat Res. 2009 Dec.
Abstract
Osteoarthritis is a degenerative disorder resulting from breakdown of articular cartilage. Previous work has shown bone morphogenic protein-7 has a potential protective effect on cartilage during the development of osteoarthritis. The purpose of this study was to determine whether bone morphogenic protein-7 could decrease the amount of cartilage degradation in preexisting osteoarthritis. The rabbit ACLT model was used as a model of osteoarthritis. Bone morphogenic protein-7 was delivered via Alzet osmotic pump to the joint 4 weeks after anterior cruciate ligament transection; thus cartilage injury was preexisting. The experimental group showed less cartilage degradation than the controls, with an average Outerbridge score of 1.9 versus 2.6 for the controls. Histomorphometry showed a trend toward less cartilage degradation in the bone morphogenic protein-7 group when compared with controls. Semiquantitative real-time polymerase chain reaction showed a considerably greater expression of aggrecan in the bone morphogenic protein-7-treated cartilage when compared with controls and less expression of matrix metalloproteinase-3 and matrix metalloproteinase-13, important catabolic mediators. The synovial tissue of the experimental group also showed considerably less expression of matrix metalloproteinase-3, matrix metalloproteinase-13, and aggrecanase. These results indicate bone morphogenic protein-7 may reduce degradation of articular cartilage in osteoarthritis.
Figures
Fig. 1A–B
This figure illustrates the calculation of percent deterioration and average defect size. (A) An actual histologic section of cartilage and (B) projection of that section for calculations are shown. (B) The red line represents the ideal cartilage surface, the blue line represents the ideal subchondral plate, and the black line represents the actual cartilage surface. The calculated area between the red and blue lines is the ideal cartilage area, whereas the calculated area between the black and blue lines is the actual cartilage area. One minus the ratio of actual area to ideal area is the percent deterioration. The average defect is calculated as an average amount of depression from the red to black lines.
Fig. 2A–B
India ink staining of (A) control and (B) BMP-7-treated knees after euthanasia shows more areas of wear and greater depths of fibrillation in the control group.
Fig. 3A–B
(A) The mean Outerbridge score of the control group (2.6) was higher than the score of the BMP-7-treated group (1.9). (B) The frequency of each Outerbridge score for control and BMP-7 groups shows a far higher percentage of severely worn condyles in the control group.
Fig. 4A–B
Sample histologic sections of (A) control group condyles and (B) BMP-7-treated condyles, taken perpendicular to the bone in the sagittal plane from the medial femoral condyles, show greater loss of articular cartilage in the control group sections.
Fig. 5A–B
(A) Histomorphometry data of the percent deterioration of cartilage in the control and BMP-7-treated groups show the percent deterioration of the control group was 30.8% (SD, 8%) of the ideal cartilage surface versus deterioration of only 23.5% (SD, 10%) for the BMP-7-treated condyles (p > 0.05). (B) Histomorphometry data of the average depression or defect of cartilage in the control and BMP-7-treated groups show the average depression of the control group was 0.141 mm (SD, 0.057 mm), whereas the BMP-7-treated group had an average depression of 0.103 mm (SD, 0.062 mm) (p > 0.05).
Fig. 6
RT-PCR data of the expression of anabolic and catabolic genes in the cartilage tissue of the control and BMP-7-treated groups are shown. The BMP-7-treated cartilage showed an increase in aggrecan expression and decrease (p < 0.05) in MMP-3 and MMP-13 expression when compared with the control group.
Fig. 7
RT-PCR data of the expression of catabolic genes in the synovial tissue of the control and BMP-7-treated groups show the BMP-7-treated synovium had a decrease (p < 0.05) in aggrecanase, MMP-3, and MMP-13 expression when compared with the control group.
Similar articles
- The protective effect of OP-1 on articular cartilage in the development of osteoarthritis.
Badlani N, Inoue A, Healey R, Coutts R, Amiel D. Badlani N, et al. Osteoarthritis Cartilage. 2008 May;16(5):600-6. doi: 10.1016/j.joca.2007.09.009. Epub 2007 Oct 31. Osteoarthritis Cartilage. 2008. PMID: 17977753 - [In vitro effect of alendronate on chondrocytes and articular cartilage and subchondral bone in rabbit anterior cruciate ligament transection model].
Hu H, Zhang L, Li B, Zhang Y, Liu X, Tian F, Cheng T, Wang Z. Hu H, et al. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009 Dec;23(12):1474-81. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009. PMID: 20073314 Chinese. - Intra-articular injections of bone morphogenetic protein-7 retard progression of existing cartilage degeneration.
Hayashi M, Muneta T, Takahashi T, Ju YJ, Tsuji K, Sekiya I. Hayashi M, et al. J Orthop Res. 2010 Nov;28(11):1502-6. doi: 10.1002/jor.21165. J Orthop Res. 2010. PMID: 20872588 - [Research progress of protective effects of alendronate on articular cartilage in osteoarthritis].
He D, Yin M, Luo Y, Wei Q. He D, et al. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012 Oct;26(10):1187-90. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012. PMID: 23167100 Review. Chinese. - The role of cytokines in osteoarthritis pathophysiology.
Fernandes JC, Martel-Pelletier J, Pelletier JP. Fernandes JC, et al. Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
Cited by
- Treatment and application of stem cells from different sources for cartilage injury: a literature review.
Wang P, Zhang S, Meng Q, Zhu P, Yuan W. Wang P, et al. Ann Transl Med. 2022 May;10(10):610. doi: 10.21037/atm-22-1715. Ann Transl Med. 2022. PMID: 35722390 Free PMC article. Review. - Targets, models and challenges in osteoarthritis research.
Thysen S, Luyten FP, Lories RJ. Thysen S, et al. Dis Model Mech. 2015 Jan;8(1):17-30. doi: 10.1242/dmm.016881. Dis Model Mech. 2015. PMID: 25561745 Free PMC article. Review. - Sustained delivery of the bone morphogenetic proteins BMP-2 and BMP-7 for cartilage repair and regeneration in osteoarthritis.
Whitty C, Pernstich C, Marris C, McCaskie A, Jones M, Henson F. Whitty C, et al. Osteoarthr Cartil Open. 2022 Feb 8;4(1):100240. doi: 10.1016/j.ocarto.2022.100240. eCollection 2022 Mar. Osteoarthr Cartil Open. 2022. PMID: 36474464 Free PMC article. - The current state of the osteoarthritis drug development pipeline: a comprehensive narrative review of the present challenges and future opportunities.
Kim H, Seo J, Lee Y, Park K, Perry TA, Arden NK, Mobasheri A, Choi H. Kim H, et al. Ther Adv Musculoskelet Dis. 2022 Dec 7;14:1759720X221085952. doi: 10.1177/1759720X221085952. eCollection 2022. Ther Adv Musculoskelet Dis. 2022. PMID: 36504595 Free PMC article. Review. - Neural EGFL like 1 as a potential pro-chondrogenic, anti-inflammatory dual-functional disease-modifying osteoarthritis drug.
Li C, Zheng Z, Ha P, Jiang W, Berthiaume EA, Lee S, Mills Z, Pan H, Chen EC, Jiang J, Culiat CT, Zhang X, Ting K, Soo C. Li C, et al. Biomaterials. 2020 Jan;226:119541. doi: 10.1016/j.biomaterials.2019.119541. Epub 2019 Oct 12. Biomaterials. 2020. PMID: 31634652 Free PMC article.
References
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/jor.1100040111', 'is_inner': False, 'url': 'https://doi.org/10.1002/jor.1100040111'}, {'type': 'PubMed', 'value': '3950812', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/3950812/'}\]}
- Amiel D, Abel MF, Kleiner JB, Lieber RL, Akeson WH. Synovial fluid nutrient delivery in the diathrial joint: an analysis of rabbit knee ligaments. J Orthop Res. 1986;4:90–95. - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/j.joca.2007.09.009', 'is_inner': False, 'url': 'https://doi.org/10.1016/j.joca.2007.09.009'}, {'type': 'PubMed', 'value': '17977753', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17977753/'}\]}
- Badlani N, Inoue A, Healey R, Coutts R, Amiel D. The protective effect of OP-1 on articular cartilage in the development of osteoarthritis. Osteoarthritis Cartilage. 2008;16:600–606. - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '9571450', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9571450/'}\]}
- Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/jor.20339', 'is_inner': False, 'url': 'https://doi.org/10.1002/jor.20339'}, {'type': 'PubMed', 'value': '17205567', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17205567/'}\]}
- Chubinskaya S, Kawakami M, Rappoport L, Matsumoto T, Migita N, Rueger DC. Anti-catabolic effect of OP-1 in chronically compressed intervertebral discs. J Orthop Res. 2007;25:517–530. - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S1357-2725(03)00035-9', 'is_inner': False, 'url': 'https://doi.org/10.1016/s1357-2725(03)00035-9'}, {'type': 'PubMed', 'value': '12798347', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12798347/'}\]}
- Chubinskaya S, Kuettner KE. Regulation of osteogenic proteins by chondrocytes. Int J Biochem Cell Biol. 2003;35:1323–1340. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical