Disruption of the neurexin 1 gene is associated with schizophrenia - PubMed (original) (raw)
. 2009 Mar 1;18(5):988-96.
doi: 10.1093/hmg/ddn351. Epub 2008 Oct 22.
Andres Ingason, Sven Cichon, Olli P H Pietiläinen, Michael R Barnes, Timothea Toulopoulou, Marco Picchioni, Evangelos Vassos, Ulrich Ettinger, Elvira Bramon, Robin Murray, Mirella Ruggeri, Sarah Tosato, Chiara Bonetto, Stacy Steinberg, Engilbert Sigurdsson, Thordur Sigmundsson, Hannes Petursson, Arnaldur Gylfason, Pall I Olason, Gudmundur Hardarsson, Gudrun A Jonsdottir, Omar Gustafsson, Ragnheidur Fossdal, Ina Giegling, Hans-Jürgen Möller, Annette M Hartmann, Per Hoffmann, Caroline Crombie, Gillian Fraser, Nicholas Walker, Jouko Lonnqvist, Jaana Suvisaari, Annamari Tuulio-Henriksson, Srdjan Djurovic, Ingrid Melle, Ole A Andreassen, Thomas Hansen, Thomas Werge, Lambertus A Kiemeney, Barbara Franke, Joris Veltman, Jacobine E Buizer-Voskamp; GROUP Investigators; Chiara Sabatti, Roel A Ophoff, Marcella Rietschel, Markus M Nöthen, Kari Stefansson, Leena Peltonen, David St Clair, Hreinn Stefansson, David A Collier
Collaborators, Affiliations
- PMID: 18945720
- PMCID: PMC2695245
- DOI: 10.1093/hmg/ddn351
Disruption of the neurexin 1 gene is associated with schizophrenia
Dan Rujescu et al. Hum Mol Genet. 2009.
Abstract
Deletions within the neurexin 1 gene (NRXN1; 2p16.3) are associated with autism and have also been reported in two families with schizophrenia. We examined NRXN1, and the closely related NRXN2 and NRXN3 genes, for copy number variants (CNVs) in 2977 schizophrenia patients and 33 746 controls from seven European populations (Iceland, Finland, Norway, Germany, The Netherlands, Italy and UK) using microarray data. We found 66 deletions and 5 duplications in NRXN1, including a de novo deletion: 12 deletions and 2 duplications occurred in schizophrenia cases (0.47%) compared to 49 and 3 (0.15%) in controls. There was no common breakpoint and the CNVs varied from 18 to 420 kb. No CNVs were found in NRXN2 or NRXN3. We performed a Cochran-Mantel-Haenszel exact test to estimate association between all CNVs and schizophrenia (P = 0.13; OR = 1.73; 95% CI 0.81-3.50). Because the penetrance of NRXN1 CNVs may vary according to the level of functional impact on the gene, we next restricted the association analysis to CNVs that disrupt exons (0.24% of cases and 0.015% of controls). These were significantly associated with a high odds ratio (P = 0.0027; OR 8.97, 95% CI 1.8-51.9). We conclude that NRXN1 deletions affecting exons confer risk of schizophrenia.
Figures
Figure 1
UCSC browser output showing the positions of the 2p16.3 CNVs from the Kirov et al. (2008), Walsh et al. (2008), Friedman et al. (2008) and Szatzmar et al. (2008) studies (blue lines) relative to the NRXN1 gene, and the CNVs discovered in the present study (schizophrenia, red lines; other psychiatric diagnoses, brown lines; controls, green lines). The majority of the discovered CNVs are deletions, asterisks indicate duplications. Markers from the Illumina 300 K and 550 K arrays, segmental duplications of >1000 bp as well as LD structure of the Hapmap CEU sample (_r_2) is also shown.
Figure 2
UCSC browser output showing the positions of exon-disrupting CNVs discovered in the study relative to the 2p16.3 CNVs from the Kirov et al. (2008), Walsh et al. (2008), Friedman et al. (2008) and Szatzmar et al. (2008) studies and known (schizophrenia, red lines; other psychiatric diagnoses, brown lines; controls, green lines; previously described CNVs, blue lines). The four putative Neurexin isoforms are shown below the deletions, along with protein domains aligned to genomic sequence.
Figure 3
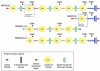
Protein domain organization of Neurexin 1 isoforms. NRXN1a contains an N-terminal signal peptide, six laminin G (LamG) domains, three epidermal growth factor-like (EGF) domains, a transmembrane domain and a short cytoplasmic domain. NRXN1b contains a different signal peptide, but shares the last LamG domain, transmembrane domain and cytoplasmic domain with NRXN1a. NRXN1a-2 shares the 2nd, 3rd, 4th and 5th LamG domains and the 2nd and 3rd EGF domains with NRXN1a, but does not contain a signal peptide or transmembrane domain. NRXN1a-3 has no signal peptide and has a truncated version of the 2nd LamG domain but shares all remaining domains with NRXN1a. The five regions in the NRXN1 gene where alternative splicing occurs, leading to insertion or deletion of amino acids is indicated by arrows (SS no. 1–5). Protein domain annotation was generated using SMART (
http://smart.embl-heidelberg.de/
), using swissprot accessions Q9ULB1 (NRXN1a) and P58400 (NRXN1b) and translations from the genbank transcript AK093260 (NRXN1a-2) and Ensembl transcript ENST00000331040 (NRXN1a-3).
Similar articles
- Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia.
Gauthier J, Siddiqui TJ, Huashan P, Yokomaku D, Hamdan FF, Champagne N, Lapointe M, Spiegelman D, Noreau A, Lafrenière RG, Fathalli F, Joober R, Krebs MO, DeLisi LE, Mottron L, Fombonne E, Michaud JL, Drapeau P, Carbonetto S, Craig AM, Rouleau GA. Gauthier J, et al. Hum Genet. 2011 Oct;130(4):563-73. doi: 10.1007/s00439-011-0975-z. Epub 2011 Mar 22. Hum Genet. 2011. PMID: 21424692 Free PMC article. - Incomplete penetrance of NRXN1 deletions in families with schizophrenia.
Todarello G, Feng N, Kolachana BS, Li C, Vakkalanka R, Bertolino A, Weinberger DR, Straub RE. Todarello G, et al. Schizophr Res. 2014 May;155(1-3):1-7. doi: 10.1016/j.schres.2014.02.023. Epub 2014 Mar 26. Schizophr Res. 2014. PMID: 24680031 - Mutation analysis of the NRXN1 gene in a Chinese autism cohort.
Liu Y, Hu Z, Xun G, Peng Y, Lu L, Xu X, Xiong Z, Xia L, Liu D, Li W, Zhao J, Xia K. Liu Y, et al. J Psychiatr Res. 2012 May;46(5):630-4. doi: 10.1016/j.jpsychires.2011.10.015. Epub 2012 Mar 9. J Psychiatr Res. 2012. PMID: 22405623 - Molecular and clinical characterization of 25 individuals with exonic deletions of NRXN1 and comprehensive review of the literature.
Béna F, Bruno DL, Eriksson M, van Ravenswaaij-Arts C, Stark Z, Dijkhuizen T, Gerkes E, Gimelli S, Ganesamoorthy D, Thuresson AC, Labalme A, Till M, Bilan F, Pasquier L, Kitzis A, Dubourgm C, Rossi M, Bottani A, Gagnebin M, Sanlaville D, Gilbert-Dussardier B, Guipponi M, van Haeringen A, Kriek M, Ruivenkamp C, Antonarakis SE, Anderlid BM, Slater HR, Schoumans J. Béna F, et al. Am J Med Genet B Neuropsychiatr Genet. 2013 Jun;162B(4):388-403. doi: 10.1002/ajmg.b.32148. Epub 2013 Mar 26. Am J Med Genet B Neuropsychiatr Genet. 2013. PMID: 23533028 Review. - The role of neurexins in schizophrenia and autistic spectrum disorder.
Reichelt AC, Rodgers RJ, Clapcote SJ. Reichelt AC, et al. Neuropharmacology. 2012 Mar;62(3):1519-26. doi: 10.1016/j.neuropharm.2011.01.024. Epub 2011 Jan 22. Neuropharmacology. 2012. PMID: 21262241 Review.
Cited by
- Exome sequencing followed by large-scale genotyping suggests a limited role for moderately rare risk factors of strong effect in schizophrenia.
Need AC, McEvoy JP, Gennarelli M, Heinzen EL, Ge D, Maia JM, Shianna KV, He M, Cirulli ET, Gumbs CE, Zhao Q, Campbell CR, Hong L, Rosenquist P, Putkonen A, Hallikainen T, Repo-Tiihonen E, Tiihonen J, Levy DL, Meltzer HY, Goldstein DB. Need AC, et al. Am J Hum Genet. 2012 Aug 10;91(2):303-12. doi: 10.1016/j.ajhg.2012.06.018. Epub 2012 Aug 2. Am J Hum Genet. 2012. PMID: 22863191 Free PMC article. - Analysis of copy number variation in Alzheimer's disease in a cohort of clinically characterized and neuropathologically verified individuals.
Swaminathan S, Huentelman MJ, Corneveaux JJ, Myers AJ, Faber KM, Foroud T, Mayeux R, Shen L, Kim S, Turk M, Hardy J, Reiman EM, Saykin AJ; Alzheimer's Disease Neuroimaging Initiative and NIA-LOAD/NCRAD Family Study Group. Swaminathan S, et al. PLoS One. 2012;7(12):e50640. doi: 10.1371/journal.pone.0050640. Epub 2012 Dec 5. PLoS One. 2012. PMID: 23227193 Free PMC article. - RIM1alpha and interacting proteins involved in presynaptic plasticity mediate prepulse inhibition and additional behaviors linked to schizophrenia.
Blundell J, Kaeser PS, Südhof TC, Powell CM. Blundell J, et al. J Neurosci. 2010 Apr 14;30(15):5326-33. doi: 10.1523/JNEUROSCI.0328-10.2010. J Neurosci. 2010. PMID: 20392954 Free PMC article. - Cognition in mouse models of schizophrenia susceptibility genes.
Arguello PA, Gogos JA. Arguello PA, et al. Schizophr Bull. 2010 Mar;36(2):289-300. doi: 10.1093/schbul/sbp153. Epub 2009 Dec 21. Schizophr Bull. 2010. PMID: 20026558 Free PMC article. Review. - REVIEW: Genome-wide findings in schizophrenia and the role of gene-environment interplay.
Van Winkel R, Esquivel G, Kenis G, Wichers M, Collip D, Peerbooms O, Rutten B, Myin-Germeys I, Van Os J. Van Winkel R, et al. CNS Neurosci Ther. 2010 Oct;16(5):e185-92. doi: 10.1111/j.1755-5949.2010.00155.x. Epub 2010 Jun 11. CNS Neurosci Ther. 2010. PMID: 20553308 Free PMC article. Review.
References
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MA, Green T, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N. Engl. J. Med. 2008;358:667–675. - PubMed
- Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, et al. Advancing paternal age and autism. Arch. Gen. Psychiatry. 2006;63:1026–1032. - PubMed
- Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, Susser ES. Advancing paternal age and the risk of schizophrenia. Arch. Gen. Psychiatry. 2001;58:361–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- 089061/WT_/Wellcome Trust/United Kingdom
- G0901310/MRC_/Medical Research Council/United Kingdom
- PDA/02/06/016/DH_/Department of Health/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical