Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis - PubMed (original) (raw)
Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis
Brigitte van Zundert et al. J Neurosci. 2008.
Abstract
Distinguishing the primary from secondary effects and compensatory mechanisms is of crucial importance in understanding adult-onset neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS). Transgenic mice that overexpress the G93A mutation of the human Cu-Zn superoxide dismutase 1 gene (hSOD1(G93A) mice) are a commonly used animal model of ALS. Whole-cell patch-clamp recordings from neurons in acute slice preparations from neonatal wild-type and hSOD1(G93A) mice were made to characterize functional changes in neuronal activity. Hypoglossal motoneurons (HMs) in postnatal day 4 (P4)-P10 hSOD1(G93A) mice displayed hyperexcitability, increased persistent Na(+) current (PC(Na)), and enhanced frequency of spontaneous excitatory and inhibitory transmission, compared with wild-type mice. These functional changes in neuronal activity are the earliest yet reported for the hSOD1(G93A) mouse, and are present 2-3 months before motoneuron degeneration and clinical symptoms appear in these mice. Changes in neuronal activity were not restricted to motoneurons: superior colliculus interneurons also displayed hyperexcitability and synaptic changes (P10-P12). Furthermore, in vivo viral-mediated GFP (green fluorescent protein) overexpression in hSOD1(G93A) HMs revealed precocious dendritic remodeling, and behavioral assays revealed transient neonatal neuromotor deficits compared with controls. These findings underscore the widespread and early onset of abnormal neural activity in this mouse model of the adult neurodegenerative disease ALS, and suggest that suppression of PC(Na) and hyperexcitability early in life might be one way to mitigate or prevent cell death in the adult CNS.
Figures
Figure 1.
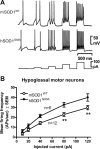
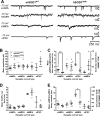
Intrinsic excitability is increased in HMs from presymptomatic hSOD1G93A ALS mice. HMs in acutely prepared brainstem slices of mSOD1WT and hSOD1G93A mice were recorded. A, Membrane potential sample traces in mSOD1WT (top) and hSOD1G93A HMs (middle) showing APs evoked by rectangular depolarizing current pulses (bottom; 10–120 pA, 300 ms, 0.5 Hz). B, Mean AP frequency plotted against injected current show that the intrinsic excitability in hSOD1G93A HMs (n = 6) is significantly increased compared with mSOD1WT HMs (n = 12). Statistical significance is indicated.
Figure 2.
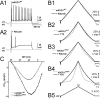
PCNa is increased in HMs from presymptomatic hSOD1G93A ALS mice. A, AP sample traces in a mSOD1WT HM without (A1) and with (A2) riluzole (20 μ
m
). Note that riluzole is unable to prevent AP generation at the current step onset but abolishes repetitive AP firing without changing subthreshold responses (gray traces). B, PCNa current traces generated by a slow (total 8 s) triangular voltage-clamp command (B5) from a holding potential of −60 to 10 mV and recorded from mSOD1WT HMs in the absence (gray traces) and presence of riluzole (20 μ
m
, black traces; B1) or TTX (500 n
m
, black traces; B2) or from hSOD1G93A HMs (riluzole, B3; TTX, B4). Note that PCNa (which is the TTX-sensitive inward current activated at voltages more than −40 mV) is significantly larger in the hSOD1G93A HM. C, Current–voltage relationship of mean PCNa current normalized to cell capacitance (current density) plotted against voltage ramp membrane potential (MP) for mSOD1WT HMs (n = 9) and hSOD1G93A HMs (n = 12), showing that the voltage dependence of PCNa is unchanged in hSOD1G93A HMs.
Figure 3.
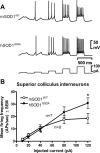
Intrinsic excitability is increased in SC interneurons from presymptomatic hSOD1G93A ALS mice. SC interneurons of acutely prepared midbrain slices of mSOD1WT and hSOD1G93A mice were recorded. A, Membrane potential sample traces in mSOD1WT (top) and hSOD1G93A SC interneurons (middle) evoked by rectangular depolarizing current pulses (bottom; 10–120 pA, 300 ms, 0.5 Hz). B, Mean AP frequency plotted against injected current show that the intrinsic excitability in hSOD1G93A SC interneurons is significantly increased compared with mSOD1WT. Statistical significance is indicated.
Figure 4.
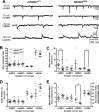
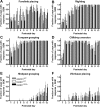
The frequency of spontaneous excitatory and inhibitory synaptic transmission is enhanced in SC interneurons from presymptomatic hSOD1G93A mice. A, Representative spontaneous (sAMPA, sGABA, and sNMDA) and quantal (mAMPA) synaptic currents traces recorded from SC interneurons of mSOD1WT (left) and hSOD1G93A (right) mice. Amplitude calibration bars are 10 pA for sAMPA, mAMPA, and sNMDA traces and 50 pA for sGABA traces. Mean amplitude (B), interevent interval (C), rise time (D), and decay time (E) for the populations of sAMPA, sGABA, mAMPA, and sNMDA events recorded from each SC interneuron from mSOD1WT (open circles) and hSOD1G93A (open triangles) mice are shown, together with the overall mean and SEM of these values for each type of current and animal genotype superimposed over the individual cell values. Note that the frequency of sAMPA (p < 0.01) and sGABA (p < 0.05) currents are significantly increased (i.e., interevent interval is decreased) in hSOD1G93A mice, whereas amplitude, rise time, and decay time of these current types are not significantly different. There are no significant differences in any parameters for mAMPA currents, whereas sNMDA currents showed only a significant decrease in decay time (p < 0.05). Student's unpaired t test was used to compare overall means of each parameter for mSOD1WT and hSOD1G93A SC interneurons.
Figure 5.
The frequency of spontaneous excitatory and inhibitory synaptic transmission is enhanced in HMs from presymptomatic hSOD1G93A mice. A, Representative spontaneous (sAMPA, sIPSC, and sNMDA) and quantal (mAMPA) synaptic currents traces recorded from HMs of mSOD1WT (left) and hSOD1G93A (right) mice. Amplitude calibration bars are 10 pA for sAMPA, mAMPA, and sNMDA traces and 50 pA for sIPSC traces. Mean amplitude (B), interevent interval (C), rise time (D), and decay time (E) for the populations of sAMPA, sIPSC, mAMPA, and sNMDA events recorded from each HM from mSOD1WT (open circles) and hSOD1G93A (open triangles) mice are shown, together with the overall mean and SEM of these values for each type of current and animal genotype superimposed over the individual cell values. Note that the frequency of sAMPA (p < 0.01) and sIPSC (p < 0.05) currents are significantly increased (i.e., interevent interval is decreased) in hSOD1G93A mice, whereas amplitude, rise time, and decay time of these current types are not significantly different. There are no significant differences in any parameters for mAMPA currents, whereas sNMDA currents showed only a significant decrease in decay time (p < 0.05). Student's unpaired t test was used to compare overall means of each parameter for mSOD1WT and hSOD1G93A HMs.
Figure 6.
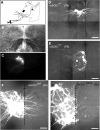
HMs dendrite retraction occurs earlier in presymptomatic hSOD1G93A mice (P6). A, Schematic of HSV-GFP p1003 (2 × 108/ml) injection in the tongue muscle at P1 to retrogradely label HMs in the ipsilateral hypoglossal nucleus (nXII). B, C, Low-magnification differential interference contrast (B) and fluorescent confocal (C) images of 350-μm-thick 4% paraformaldehyde-fixed slices of nXII showing GFP expression in HMs. D, E, Higher-magnification images showing GFP-positive mSOD1WT HMs, with labeled dendrites crossing midline (dashed line) at P6 (D) but not present at P9 (E). F, G, At P6, hSOD1G93A HMs (G) had fewer midline-crossing dendrites than mSOD1WT motoneurons (F). Scale bars: D, E, 100 μm; F, G, 50 μm.
Figure 7.
Transient delays in development of gross locomotor abilities in presymptomatic hSOD1G93A mice are present at P2–P4. The fraction of pups displaying forelimb placing (A, at P3–P4 only) or righting (B, at P2 only) responses was lower in hSOD1G93A than in mSOD1WT and hSOD1WT mice (p < 0.05, χ2 test), whereas development of forepaw (C) and hindpaw (E) grasping, cliff-drop aversion (D), and vibrissae placing (F) were unaltered.
Similar articles
- Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.
Jaarsma D, Haasdijk ED, Grashorn JA, Hawkins R, van Duijn W, Verspaget HW, London J, Holstege JC. Jaarsma D, et al. Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261 - Inhibitory synaptic regulation of motoneurons: a new target of disease mechanisms in amyotrophic lateral sclerosis.
Martin LJ, Chang Q. Martin LJ, et al. Mol Neurobiol. 2012 Feb;45(1):30-42. doi: 10.1007/s12035-011-8217-x. Epub 2011 Nov 10. Mol Neurobiol. 2012. PMID: 22072396 Free PMC article. Review. - Embryonic alteration of motoneuronal morphology induces hyperexcitability in the mouse model of amyotrophic lateral sclerosis.
Martin E, Cazenave W, Cattaert D, Branchereau P. Martin E, et al. Neurobiol Dis. 2013 Jun;54:116-26. doi: 10.1016/j.nbd.2013.02.011. Epub 2013 Mar 4. Neurobiol Dis. 2013. PMID: 23466698 - eGFP expression under UCHL1 promoter genetically labels corticospinal motor neurons and a subpopulation of degeneration-resistant spinal motor neurons in an ALS mouse model.
Yasvoina MV, Genç B, Jara JH, Sheets PL, Quinlan KA, Milosevic A, Shepherd GM, Heckman CJ, Özdinler PH. Yasvoina MV, et al. J Neurosci. 2013 May 1;33(18):7890-904. doi: 10.1523/JNEUROSCI.2787-12.2013. J Neurosci. 2013. PMID: 23637180 Free PMC article. - Early abnormalities in transgenic mouse models of amyotrophic lateral sclerosis.
Durand J, Amendola J, Bories C, Lamotte d'Incamps B. Durand J, et al. J Physiol Paris. 2006 Mar-May;99(2-3):211-20. doi: 10.1016/j.jphysparis.2005.12.014. Epub 2006 Jan 30. J Physiol Paris. 2006. PMID: 16448809 Review.
Cited by
- From Physiological Properties to Selective Vulnerability of Motor Units in Amyotrophic Lateral Sclerosis.
Bączyk M, Manuel M, Roselli F, Zytnicki D. Bączyk M, et al. Adv Neurobiol. 2022;28:375-394. doi: 10.1007/978-3-031-07167-6_15. Adv Neurobiol. 2022. PMID: 36066833 - Amyotrophic lateral sclerosis mutant TDP-43 may cause synaptic dysfunction through altered dendritic spine function.
Jiang T, Handley E, Brizuela M, Dawkins E, Lewis KEA, Clark RM, Dickson TC, Blizzard CA. Jiang T, et al. Dis Model Mech. 2019 May 17;12(5):dmm038109. doi: 10.1242/dmm.038109. Dis Model Mech. 2019. PMID: 31036551 Free PMC article. - Abnormal Upregulation of GPR17 Receptor Contributes to Oligodendrocyte Dysfunction in SOD1 G93A Mice.
Bonfanti E, Bonifacino T, Raffaele S, Milanese M, Morgante E, Bonanno G, Abbracchio MP, Fumagalli M. Bonfanti E, et al. Int J Mol Sci. 2020 Mar 31;21(7):2395. doi: 10.3390/ijms21072395. Int J Mol Sci. 2020. PMID: 32244295 Free PMC article. - Identification and therapeutic rescue of autophagosome and glutamate receptor defects in C9ORF72 and sporadic ALS neurons.
Shi Y, Hung ST, Rocha G, Lin S, Linares GR, Staats KA, Seah C, Wang Y, Chickering M, Lai J, Sugawara T, Sagare AP, Zlokovic BV, Ichida JK. Shi Y, et al. JCI Insight. 2019 Jul 16;5(15):e127736. doi: 10.1172/jci.insight.127736. JCI Insight. 2019. PMID: 31310593 Free PMC article. - The Electrophysiological Determinants of Corticospinal Motor Neuron Vulnerability in ALS.
Jara JH, Sheets PL, Nigro MJ, Perić M, Brooks C, Heller DB, Martina M, Andjus PR, Ozdinler PH. Jara JH, et al. Front Mol Neurosci. 2020 May 19;13:73. doi: 10.3389/fnmol.2020.00073. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32508590 Free PMC article.
References
- Aamodt SM, Shi J, Colonnese MT, Veras W, Constantine-Paton M. Chronic NMDA exposure accelerates development of GABAergic inhibition in the superior colliculus. J Neurophysiol. 2000;83:1580–1591. - PubMed
- Amendola J, Verrier B, Roubertoux P, Durand J. Altered sensorimotor development in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2004;20:2822–2826. - PubMed
- Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ current in mouse neocortical slices. J Neurophysiol. 1998;80:1547–1551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY014074/EY/NEI NIH HHS/United States
- R01 NS036640-03/NS/NINDS NIH HHS/United States
- P01 AG012992-10/AG/NIA NIH HHS/United States
- R01 EY006039/EY/NEI NIH HHS/United States
- P01 AG012992/AG/NIA NIH HHS/United States
- R01 EY006039-26/EY/NEI NIH HHS/United States
- EY06039/EY/NEI NIH HHS/United States
- R37 EY006039/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous