A bacterial control circuit integrates polar localization and proteolysis of key regulatory proteins with a phospho-signaling cascade - PubMed (original) (raw)
A bacterial control circuit integrates polar localization and proteolysis of key regulatory proteins with a phospho-signaling cascade
Antonio A Iniesta et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Dynamic protein localization is an integral component of the regulatory circuit that drives the Caulobacter cell cycle. The ClpXP protease is localized to the Caulobacter cell pole, where it catalyzes the degradation of the CtrA master regulator at specific times in the cell cycle. Clearance of active CtrA at the G1/S transition allows the initiation of DNA replication and cell-cycle progression. The polar localization of ClpXP is dependent on the polar positioning of the CpdR single-domain response regulator. Only the unphosphorylated form of CpdR localizes and activates ClpXP. We demonstrate that another single domain response regulator, DivK, promotes the polar accumulation of unphosphorylated CpdR and that CpdR is subsequently degraded at the cell pole by the localized ClpXP protease. Thus, CpdR function is regulated by a feedback loop that incorporates its differential phosphorylation, the transient polar localization and activity of the ClpXP protease, and the clearance of the CpdR by polar ClpXP that, in turn, releases ClpXP from the pole relieving the degradation of CtrA. CtrA approximately P then accumulates and activates the transcription of cpdR, completing the regulatory loop, establishing an integrated network that controls a robust cell-cycle transition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
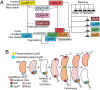
Fig. 1.
Model for CpdR and CtrA regulation (A) and cell cycle progression (B). (A) Continuous and dashed lines indicate direct or possible indirect effects, respectively. The black boxes represent CtrA binding sites. (B) The differential spatial distribution of multiple regulatory elements that drive cell-cycle progression are shown. PD, predivisional cell, ST, stalked cell; SW, swarmer cell.
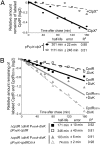
Fig. 2.
The ClpX-mediated degradation of CpdR is dependent on its phosphorylation state and on DivK. (A) WT cells harboring the plasmid pP_xylX_-clpX* (14) were grown in M2G medium in the absence of xylose. Culture samples were incubated for 4 h either in the absence (solid line) or in the presence of xylose to allow clpX* induction (dashed line). (B) Pulse–chase experiments showing the half-lives of CpdR (black lines) and CpdRD51A (gray lines) in the presence (solid lines) or absence (dashed lines) of DivK. Each strain was incubated in the presence of xylose to induce cpdR or cpdR D51A and in the presence or absence of vanillate (presence or absence of DivK, respectively). R2 is the regression coefficient.
Fig. 3.
(A) CpdR (solid line), but not CpdRD51A (dashed line) accumulates in the absence of DivK. Indicated strains were incubated in the absence of vanillate (absence of DivK), but in the presence of xylose (presence of CpdR or CpdRD51A). Samples were taken every 2 h for Western blot analysis using anti-CpdR antibodies, and the amounts of CpdR or CpdRD51A were determined. Immunoblot using anti-DivK antibodies showing the levels of DivK accumulation after DivK depletion by vanillate removal (Bottom). Cultures of strain Δ_divK_ P_vanA_-divK were grown in M2G media in presence of vanillate (presence of DivK). After vanillate removal, two aliquots of the same culture were grown in the presence or absence of vanillate. Western blot analysis was performed on samples collected from each culture every 2 h over the duration of the experiment. DivK was significantly decreased after 2 h of incubation in the absence of vanillate, and was undetectable after 6 h. (B) DivK controls the polar localization of CpdR. Differential interference contrast (DIC) and fluorescence images of CpdR-YFP or CpdRD51A-YFP in the presence or absence of DivK. Cultures from the indicated strains were grown in M2G media with vanillate (presence of DivK). After vanillate removal, culture samples were incubated in either the presence or absence of vanillate. After 8 h of DivK depletion, cells were induced with xylose (for cpdR-yfp or cpdR D51A -yfp expression) for 1 h. The proportion of cells showing at least one polar YFP fluorescence focus (white arrows) and number of cells observed are indicated.
Fig. 4.
DivK controls the polar localization of ClpX and CtrA. (A and B) DIC and fluorescence images of ClpX-GFP in the presence of CpdR or CpdRD51A, and in the presence or absence of DivK. Cultures from the indicated strains were grown in M2G medium with vanillate, to induce divK expression, and aliquots were incubated in either the presence or absence of vanillate. After 8 h of DivK depletion, cells were induced with xylose (for clpX-gfp and cpdR or cpdR D51A expression) for 2 h. Proportion of cells showing at least one polar ClpX-GFP fluorescence focus (white arrows) and number of cells observed are indicated. (C and D) DIC and fluorescence images of YFP-CtrA-RD-15 in the presence of CpdR or CpdRD51A, and in the presence or absence of DivK. After 8 h of DivK depletion, cells from the indicated strains were induced with xylose (for yfp-ctrA- RD-15 and cpdR or cpdR D51A expression) for 2 h. Polar YFP-CtrA-RD-15 fluorescence foci are indicated (white arrows).
Fig. 5.
The presence of the unphosphorylatable CpdRD51A bypasses the requirement of DivK for cell survival. (A) Four indicated strains were grown in PYE media in the presence of vanillate (divK expression) and xylose (cpdR or cpdR D51A expression) to an O.D.660 of 0.6. The cultures were serially diluted, and 5 μl of each sample was spotted onto PYE plates containing no vanillate but xylose (Left), and no vanillate but glucose (Right). (B) Cells from the strains Δ_cpdR_ Δ_divK_ P_vanA_-divK pP_xylX_-cpdR (i–v) and Δ_cpdR_ Δ_divK_ P_vanA_-divK pP_xylX_-cpdR D51A (vi–x) were grown in M2G media in the presence of vanillate (presence of DivK) and xylose (presence of CpdR or CpdRD51A). Culture samples were washed and incubated in M2G media in the presence or absence of vanillate or xylose before microscopic observation. All cultures were kept in exponential phase growth for the entire experiment.
Similar articles
- A phospho-signaling pathway controls the localization and activity of a protease complex critical for bacterial cell cycle progression.
Iniesta AA, McGrath PT, Reisenauer A, McAdams HH, Shapiro L. Iniesta AA, et al. Proc Natl Acad Sci U S A. 2006 Jul 18;103(29):10935-40. doi: 10.1073/pnas.0604554103. Epub 2006 Jul 7. Proc Natl Acad Sci U S A. 2006. PMID: 16829582 Free PMC article. - Polar Localization Hub Protein PopZ Restrains Adaptor-Dependent ClpXP Proteolysis in Caulobacter crescentus.
Joshi KK, Battle CM, Chien P. Joshi KK, et al. J Bacteriol. 2018 Sep 24;200(20):e00221-18. doi: 10.1128/JB.00221-18. Print 2018 Oct 15. J Bacteriol. 2018. PMID: 30082457 Free PMC article. - A dynamically localized protease complex and a polar specificity factor control a cell cycle master regulator.
McGrath PT, Iniesta AA, Ryan KR, Shapiro L, McAdams HH. McGrath PT, et al. Cell. 2006 Feb 10;124(3):535-47. doi: 10.1016/j.cell.2005.12.033. Cell. 2006. PMID: 16469700 - Spatial and temporal control of differentiation and cell cycle progression in Caulobacter crescentus.
Ausmees N, Jacobs-Wagner C. Ausmees N, et al. Annu Rev Microbiol. 2003;57:225-47. doi: 10.1146/annurev.micro.57.030502.091006. Annu Rev Microbiol. 2003. PMID: 14527278 Review. - Who's in charge here? Regulating cell cycle regulators.
Bowers LM, Shapland EB, Ryan KR. Bowers LM, et al. Curr Opin Microbiol. 2008 Dec;11(6):547-52. doi: 10.1016/j.mib.2008.09.019. Epub 2008 Nov 3. Curr Opin Microbiol. 2008. PMID: 18955157 Review.
Cited by
- Influence of the Heme Nitric Oxide/Oxygen Binding Protein (H-NOX) on Cell Cycle Regulation in Caulobacter crescentus.
Lee-Lopez C, Islam MS, Meléndez AB, Yukl ET. Lee-Lopez C, et al. Mol Cell Proteomics. 2023 Dec;22(12):100679. doi: 10.1016/j.mcpro.2023.100679. Epub 2023 Nov 17. Mol Cell Proteomics. 2023. PMID: 37979947 Free PMC article. - Degron-mediated proteolysis of CrhR-like DEAD-box RNA helicases in cyanobacteria.
Whitman BT, Murray CRA, Whitford DS, Paul SS, Fahlman RP, Glover MJN, Owttrim GW. Whitman BT, et al. J Biol Chem. 2022 May;298(5):101925. doi: 10.1016/j.jbc.2022.101925. Epub 2022 Apr 10. J Biol Chem. 2022. PMID: 35413287 Free PMC article. - Proteolysis dependent cell cycle regulation in Caulobacter crescentus.
Fatima NI, Fazili KM, Bhat NH. Fatima NI, et al. Cell Div. 2022 Apr 1;17(1):3. doi: 10.1186/s13008-022-00078-z. Cell Div. 2022. PMID: 35365160 Free PMC article. Review. - Modeling the temporal dynamics of master regulators and CtrA proteolysis in Caulobacter crescentus cell cycle.
Xu C, Hollis H, Dai M, Yao X, Watson LT, Cao Y, Chen M. Xu C, et al. PLoS Comput Biol. 2022 Jan 28;18(1):e1009847. doi: 10.1371/journal.pcbi.1009847. eCollection 2022 Jan. PLoS Comput Biol. 2022. PMID: 35089921 Free PMC article. - A localized adaptor protein performs distinct functions at the Caulobacter cell poles.
Wang J, Moerner WE, Shapiro L. Wang J, et al. Proc Natl Acad Sci U S A. 2021 Mar 30;118(13):e2024705118. doi: 10.1073/pnas.2024705118. Proc Natl Acad Sci U S A. 2021. PMID: 33753507 Free PMC article.
References
- Goley ED, Iniesta AA, Shapiro L. Cell cycle regulation in Caulobacter: Location, location, location. J Cell Sci. 2007;120:3501–3507. - PubMed
- McAdams HH, Shapiro L. A bacterial cell-cycle regulatory network operating in time and space. Science. 2003;301:1874–1877. - PubMed
- Domian IJ, Quon KC, Shapiro L. Cell type specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90:415–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources