From cells to organs: building polarized tissue - PubMed (original) (raw)
Review
From cells to organs: building polarized tissue
David M Bryant et al. Nat Rev Mol Cell Biol. 2008 Nov.
Abstract
How do animal cells assemble into tissues and organs? A diverse array of tissue structures and shapes can be formed by organizing groups of cells into different polarized arrangements and by coordinating their polarity in space and time. Conserved design principles underlying this diversity are emerging from studies of model organisms and tissues. We discuss how conserved polarity complexes, signalling networks, transcription factors, membrane-trafficking pathways, mechanisms for forming lumens in tubes and other hollow structures, and transitions between different types of polarity, such as between epithelial and mesenchymal cells, are used in similar and iterative manners to build all tissues.
Figures
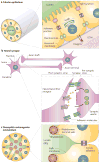
Figure 1. Cell polarization in diverse tissue types
a | Epithelial tubes are comprised of tightly adhering cells that display strong apico–basal polarity. Lateral membranes possess desmosomes, adherens junctions and tight junctions (TJs), providing cell–cell adhesion and diffusion barriers. Basal membranes interact with underlying basement membrane and extracellular matrix (ECM). Apical membranes are specialized for absorption and secretion, such as for electrolytes, milk or O2. b | Neurons polarize to form a soma (cell body), an axon shaft, an axon terminal and dendrites. Neural synapses contain adhesion molecules for stabilization of the interaction between cells. The synapse provides a specialized region for neurotransmission to occur, through polarized targeting and uptake of neurotransmitters. c | The Drosophila melanogaster retina contains ommatidia made up of tubular neuroepithelia surrounding a central lumen (or interrhabdomeral space (IRS)). Cells are not radially symmetric in the tube but nevertheless follow a defined, polarized pattern. They have typical epithelial junctions (zonula adherens at the most apico–lateral region in D. melanogaster) and a subapical actin-dense network (the rhabdomere terminal web), but their apical surfaces are specialized into two domains; the stalk membrane and the light-sensing rhabdomeres, which are specialized microvilli.
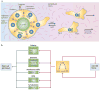
Figure 2. EMT and MET in tissue morphogenesis
a | Epithelial cells from tubes undergoing epithelial–mesenchymal transition (EMT) lose apico–basal polarity, downregulate cell–cell adhesion, change their cytoskeleton composition and invade into the extracellular matrix (ECM) at areas where the basement membrane has broken down. As cells adopt a mesenchymal state, they may also be polarized, displaying front–back polarization and Golgi orientation towards the leading edge (front) during migration,. Conversely, during mesenchymal–epithelial transition (MET), cells develop apico–basal polarity, express epithelial-specific proteins, form stable adhesions and generate luminal structures. b | Transforming growth factor-β (TGFβ) (and other morphogens) can induce EMT by inducing Snail and the ZEB family of transcription factors (which can also crosstalk). Snail and ZEB directly repress the expression of numerous proteins that are involved in epithelial polarization, including polarity complexes,, cell–cell junctions, the ECM and the cytoskeleton. Snail-1 can also alter membrane trafficking, such as by direct repression of RAB25 (REF. 32), a small GTPase that is involved in apical membrane trafficking (although, paradoxically, RAB25 is overexpressed in some tumour types131). The miR-200 family of microRNAs promotes epithelial polarity and can induce MET by inhibiting translation of ZEB1, thereby blocking induction of EMT,.
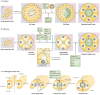
Figure 3. Cavitation, hollowing and membrane repulsion as lumen-forming mechanisms
a | During cavitation, groups of cells proliferate to form a cell mass. Apoptosis of non-extracellular matrix (ECM)-contacting inner cells results in lumen and polarized tube or cyst formation. Correct lumen formation requires the PAR and Scribble complexes, which modulate cell proliferation. Apoptosis requires BCL2-family members (BCL2 and BIM) and is inhibited by proliferation-inducing oncoproteins (ERBB2, cyclin D1 and HPV16 E7), causing luminal filling,,. b | During hollowing, polarity establishment and orientation requires laminin–β1-integrin to signal RAC1 (REFS 12,37), and is inhibited by RhoA–ROCK1 signalling. Intracellular vesicles (varying in size in different systems71) containing apical membrane components and endocytosis- and/or _trans_-Golgi-derived material are delivered to regions between cells,,. This delivery depends on PTEN-mediated segregation of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) to nascent apical regions, recruiting annexin-2 and, in turn, the CDC42–aPKC–PAR6 complex,. Once rudimentary lumens are formed (there may be multiple such lumens), tight junctions,,, pump proteins,, the Crumbs complex,, the exocyst (Sec10 (REF. 136)) and possibly ezrin, promote formation of a single, expanded lumen. c | During Drosophila melanogaster cardiac tube formation, two rows of myoendothelial cells line up along the midline. Membrane processes extend and join between cells on either side of the midline, first at the ventral-most and then at the dorsal-most regions between cells. Resultant junctions containing PAR3 (Bazooka (Baz)), DE-cadherin (Shotgun (Shg)), β-catenin (Armadillo (Arm)) and Ena allow an enclosed lumen. Slit signalling to Robo at lumen surfaces, apparently regulated by dystroglycan (Dg), prevents extended adhesive contacts between cells (membrane repulsion), allowing lumens to form,.
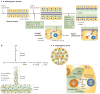
Figure 4. Membrane traffic and apical extracellular matrix secretion during lumen formation and expansion
a | In the embryonic Drosophila melanogaster trachea an initially narrow lumen is expanded during a rapid burst of Molecular Cell Biology secretion at the apical membrane, dependent on the COPI and COPII vesicle transport complexes,. Chitin fibrils in the lumen (made by chitin synthetases) signal to underlying cells to organize luminal diameter before being subsequently remodelled into tracheal cuticle (reviewed in REF. 94). Clathrin-dependent endocytic activity at the apical surface directs luminal material to early endosomes (EE) and then to late endosomes (LE) for degradation, thereby clearing the lumen for gas entry. Correct luminal secretion and expansion requires functional septate junctions. b | Part of the D. melanogaster airway is divided into the dorsal trunk and dorsal branches, which sprout from the trunk. Wingless (Wg) signalling in the trunk induces the Spalt (SAL) transcription factor, which promotes DE-cadherin recycling through induction of the RIP11–RAB11 membrane-trafficking complex; lumens are formed by intercellular junctions between multiple cells. Decapentaplegic (Dpp) signalling in branches inhibits SAL expression, through the repressor Knirps (KNI), downregulating the RIP11–RAB11 complex and leading to formation of autocellular junctions and lumens in individual cells. c | During ommatidium formation in the D. melanogaster retina, a tripartite complex of RAB11, RIP11 and the motor protein myosin V (MyoV) regulates transport of cargo to the rhabdomere, and is required for correct ommatidia organization. RAB11 interacts with the exocyst complex, which also regulates apical transport to the rhabdomere. Luminal secretion of the proteoglycan eyes shut (EYS) is required for expansion of the interrhabdomeral space (IRS), apparently occurring in an exocyst-independent manner. ZA, zonula adherens.
Comment in
- Outside in: inversion of cell polarity controls epithelial lumen formation.
Davis GE, Cleaver OB. Davis GE, et al. Dev Cell. 2014 Oct 27;31(2):140-2. doi: 10.1016/j.devcel.2014.10.011. Dev Cell. 2014. PMID: 25373773 Free PMC article.
Similar articles
- Cells into tubes: Molecular and physical principles underlying lumen formation in tubular organs.
Camelo C, Luschnig S. Camelo C, et al. Curr Top Dev Biol. 2021;143:37-74. doi: 10.1016/bs.ctdb.2020.09.002. Epub 2020 Oct 20. Curr Top Dev Biol. 2021. PMID: 33820625 Review. - Cell interactions and patterned intercalations shape and link epithelial tubes in C. elegans.
Rasmussen JP, Feldman JL, Reddy SS, Priess JR. Rasmussen JP, et al. PLoS Genet. 2013;9(9):e1003772. doi: 10.1371/journal.pgen.1003772. Epub 2013 Sep 5. PLoS Genet. 2013. PMID: 24039608 Free PMC article. - Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis.
Martín-Belmonte F, Yu W, Rodríguez-Fraticelli AE, Ewald AJ, Werb Z, Alonso MA, Mostov K. Martín-Belmonte F, et al. Curr Biol. 2008 Apr 8;18(7):507-13. doi: 10.1016/j.cub.2008.02.076. Curr Biol. 2008. PMID: 18394894 Free PMC article. - Deciphering the interplay between autophagy and polarity in epithelial tubulogenesis.
Alfonso-Pérez T, Baonza G, Herranz G, Martín-Belmonte F. Alfonso-Pérez T, et al. Semin Cell Dev Biol. 2022 Nov;131:160-172. doi: 10.1016/j.semcdb.2022.05.015. Epub 2022 May 28. Semin Cell Dev Biol. 2022. PMID: 35641407 Review. - Luminal signalling links cell communication to tissue architecture during organogenesis.
Durdu S, Iskar M, Revenu C, Schieber N, Kunze A, Bork P, Schwab Y, Gilmour D. Durdu S, et al. Nature. 2014 Nov 6;515(7525):120-4. doi: 10.1038/nature13852. Epub 2014 Oct 22. Nature. 2014. PMID: 25337877
Cited by
- EB1-recruited microtubule +TIP complexes coordinate protrusion dynamics during 3D epithelial remodeling.
Gierke S, Wittmann T. Gierke S, et al. Curr Biol. 2012 May 8;22(9):753-62. doi: 10.1016/j.cub.2012.02.069. Epub 2012 Apr 5. Curr Biol. 2012. PMID: 22483942 Free PMC article. - Bone morphogenetic proteins regulate hinge point formation during neural tube closure by dynamic modulation of apicobasal polarity.
Eom DS, Amarnath S, Fogel JL, Agarwala S. Eom DS, et al. Birth Defects Res A Clin Mol Teratol. 2012 Oct;94(10):804-16. doi: 10.1002/bdra.23052. Epub 2012 Aug 3. Birth Defects Res A Clin Mol Teratol. 2012. PMID: 22865775 Free PMC article. - CDC42 is required for structural patterning of the lung during development.
Wan H, Liu C, Wert SE, Xu W, Liao Y, Zheng Y, Whitsett JA. Wan H, et al. Dev Biol. 2013 Feb 1;374(1):46-57. doi: 10.1016/j.ydbio.2012.11.030. Epub 2012 Dec 5. Dev Biol. 2013. PMID: 23219958 Free PMC article. - Real-time sensing of cell morphology by infrared waveguide spectroscopy.
Yashunsky V, Marciano T, Lirtsman V, Golosovsky M, Davidov D, Aroeti B. Yashunsky V, et al. PLoS One. 2012;7(10):e48454. doi: 10.1371/journal.pone.0048454. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23119025 Free PMC article. - Rab27 effector Slp2-a transports the apical signaling molecule podocalyxin to the apical surface of MDCK II cells and regulates claudin-2 expression.
Yasuda T, Saegusa C, Kamakura S, Sumimoto H, Fukuda M. Yasuda T, et al. Mol Biol Cell. 2012 Aug;23(16):3229-39. doi: 10.1091/mbc.E12-02-0104. Epub 2012 Jul 5. Mol Biol Cell. 2012. PMID: 22767581 Free PMC article.
References
- O’Brien LE, Zegers MM, Mostov KE. Building epithelial architecture: insights from three-dimensional culture models. Nature Rev Mol Cell Biol. 2002;3:531–537. - PubMed
- Lecuit T, Le Goff L. Orchestrating size and shape during morphogenesis. Nature. 2007;450:189–192. - PubMed
- Alberts B. Molecular Biology Of The Cell. Garland Science; New York: 2008.
Publication types
MeSH terms
Grants and funding
- P01 AI053194/AI/NIAID NIH HHS/United States
- R01 DK074398-02/DK/NIDDK NIH HHS/United States
- P01 AI053194-06A1/AI/NIAID NIH HHS/United States
- R37 AI025144-22/AI/NIAID NIH HHS/United States
- R37 AI025144/AI/NIAID NIH HHS/United States
- R01 DK074398/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources