Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice - PubMed (original) (raw)
Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice
Jennifer J Manning-Tobin et al. Arterioscler Thromb Vasc Biol. 2009 Jan.
Abstract
Objective: The scavenger receptors SR-A and CD36 have been implicated in macrophage foam cell formation during atherogenesis and in the regulation of inflammatory signaling pathways, including those leading to lesional macrophage apoptosis and plaque necrosis. To test the impact of deleting these receptors, we generated Apoe(-/-) mice lacking both SR-A and CD36 and fed them a Western diet for 12 weeks.
Methods and results: We analyzed atheroma in mice, assessing lesion size, foam cell formation, inflammatory gene expression, apoptosis, and necrotic core formation. Aortic root atherosclerosis in Apoe(-/-)Cd36(-/-)Msr1(-/-) mice, as assessed by morphometry, electron microscopy, and immunohistochemistry, showed no decrease in lesion area or in vivo foam cell formation when compared to Apoe(-/-) mice. However, Apoe(-/-)Cd36(-/-)Msr1(-/-) lesions showed reduced expression of inflammatory genes and morphological analysis revealed a approximately 30% decrease in macrophage apoptosis and a striking approximately 50% decrease in plaque necrosis in aortic root lesions of these mice.
Conclusions: Although targeted deletion of SR-A and CD36 does not abrogate macrophage foam cell formation or substantially reduce atherosclerotic lesion area in Apoe(-/-) mice, loss of these pathways does reduce progression to more advanced necrotic lesions. These data suggest that targeted inhibition of these pathways in vivo may reduce lesional inflammation and promote plaque stability.
Figures
Figure 1
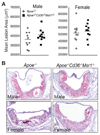
Disruption of Cd36 and Msr1 does not alter the extent of aortic root atherosclerosis in hyperlipidemic _Apoe_−/− mice. Aortic root atherosclerotic lesion area was quantified from 12 serial sections per mouse. Data are presented as (A) the mean lesion area of individual mice. N.S.; ANOVA. B, Representative photographs of oil red O–stained aortic root lesions. Magnification, × 100.
Figure 2
Immunohistochemical characterization of aortic root atherosclerosis. Aortic root lesions were stained for macrophage (MOMA-2) or smooth muscle cell content (α-smooth muscle actin) and quantified as a percentage of lesion area (±SEM) using 3 sections per mouse. N.S.; Student t test. Magnification, ×100 (upper panel, middle panel), × 250 (B, lower panel).
Figure 3
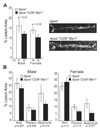
Morphometric analysis of lesion area measured in the aorta en face. A, Total aortic lesion area in male and female _Apoe_−/− and _Apoe_−/− _Cd36_−/− _Msr1_−/− mice and representative photographs of en face aortae. B, Regional analysis of lesion distribution in the aorta. Data are expressed as mean % lesion area ± SEM.
Figure 4
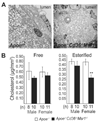
Analysis of lesional foam cell and cholesterol accumulation in the absence of CD36 and SR-A. A, Electron photomicrographs demonstrating intracellular lipid accumulation in the intima of aortic root lesions of _Apoe_−/− and _Apoe_−/− _Cd36_−/− _Msr1_−/− mice. Magnification, × 5000. B, Aortic free and esterified cholesterol content. **P<0.005; ANOVA.
Figure 5
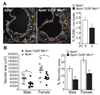
Reduced apoptotic cell death and necrotic core formation in lesions of _Apoe_−/− _Cd36_−/− _Msr1_−/− mice. A, Lesional apoptotic cells in aortic root lesions of female mice were detected by TUNEL staining. Representative fluorescent images (×100 magnification) and quantitative data are shown. Red, TUNEL positive nuclei; white, DAPI-stained nuclei. *P<0.05; Student t test. B, Quantitative analysis of necrotic core size relative to total lesion area; n=9 to 11 per group; **P<0.005, *P<0.05; Student t test.
Similar articles
- Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice.
Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Moore KJ, et al. J Clin Invest. 2005 Aug;115(8):2192-201. doi: 10.1172/JCI24061. J Clin Invest. 2005. PMID: 16075060 Free PMC article. - SPA Promotes Atherosclerosis Through Mediating Macrophage Foam Cell Formation-Brief Report.
King SD, Cai D, Pillay A, Fraunfelder MM, Allen LH, Chen SY. King SD, et al. Arterioscler Thromb Vasc Biol. 2024 Nov;44(11):e277-e287. doi: 10.1161/ATVBAHA.124.321460. Epub 2024 Oct 3. Arterioscler Thromb Vasc Biol. 2024. PMID: 39360411 - Vav Guanine nucleotide exchange factors regulate atherosclerotic lesion development in mice.
Rahaman SO, Li W, Silverstein RL. Rahaman SO, et al. Arterioscler Thromb Vasc Biol. 2013 Sep;33(9):2053-7. doi: 10.1161/ATVBAHA.113.301414. Epub 2013 Jul 3. Arterioscler Thromb Vasc Biol. 2013. PMID: 23825362 Free PMC article. - Transgenic mouse models to study the role of the macrophage scavenger receptor class A in atherosclerosis.
De Winther MP, Gijbels MJ, Van Dijk KW, Havekes LM, Hofker MH. De Winther MP, et al. Int J Tissue React. 2000;22(2-3):85-91. Int J Tissue React. 2000. PMID: 10937358 Review. - Foam cells in atherosclerosis.
Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. Yu XH, et al. Clin Chim Acta. 2013 Sep 23;424:245-52. doi: 10.1016/j.cca.2013.06.006. Epub 2013 Jun 16. Clin Chim Acta. 2013. PMID: 23782937 Review.
Cited by
- Receptor-independent fluid-phase macropinocytosis promotes arterial foam cell formation and atherosclerosis.
Lin HP, Singla B, Ahn W, Ghoshal P, Blahove M, Cherian-Shaw M, Chen A, Haller A, Hui DY, Dong K, Zhou J, White J, Stranahan AM, Jasztal A, Lucas R, Stansfield BK, Fulton D, Chlopicki S, Csányi G. Lin HP, et al. Sci Transl Med. 2022 Sep 21;14(663):eadd2376. doi: 10.1126/scitranslmed.add2376. Epub 2022 Sep 21. Sci Transl Med. 2022. PMID: 36130017 Free PMC article. - Calpain-6 confers atherogenicity to macrophages by dysregulating pre-mRNA splicing.
Miyazaki T, Tonami K, Hata S, Aiuchi T, Ohnishi K, Lei XF, Kim-Kaneyama JR, Takeya M, Itabe H, Sorimachi H, Kurihara H, Miyazaki A. Miyazaki T, et al. J Clin Invest. 2016 Sep 1;126(9):3417-32. doi: 10.1172/JCI85880. Epub 2016 Aug 15. J Clin Invest. 2016. PMID: 27525442 Free PMC article. - Functional alterations of myeloid cell subsets in hyperlipidaemia: relevance for atherosclerosis.
Soehnlein O, Drechsler M, Hristov M, Weber C. Soehnlein O, et al. J Cell Mol Med. 2009 Nov-Dec;13(11-12):4293-303. doi: 10.1111/j.1582-4934.2009.00965.x. Epub 2009 Nov 9. J Cell Mol Med. 2009. PMID: 19900213 Free PMC article. Review. - Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways.
Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Timmins JM, et al. J Clin Invest. 2009 Oct;119(10):2925-41. doi: 10.1172/JCI38857. Epub 2009 Sep 8. J Clin Invest. 2009. PMID: 19741297 Free PMC article. - Regression and stabilization of advanced murine atherosclerotic lesions: a comparison of LDL lowering and HDL raising gene transfer strategies.
Van Craeyveld E, Gordts SC, Nefyodova E, Jacobs F, De Geest B. Van Craeyveld E, et al. J Mol Med (Berl). 2011 Jun;89(6):555-67. doi: 10.1007/s00109-011-0722-x. Epub 2011 Jan 21. J Mol Med (Berl). 2011. PMID: 21249329 Free PMC article.
References
- Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. - PubMed
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. - PubMed
- Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL75662/HL/NHLBI NIH HHS/United States
- HL45098/HL/NHLBI NIH HHS/United States
- R01 HL057560/HL/NHLBI NIH HHS/United States
- AG20255/AG/NIA NIH HHS/United States
- R01 HL045098/HL/NHLBI NIH HHS/United States
- HL57560/HL/NHLBI NIH HHS/United States
- R24 RR020345/RR/NCRR NIH HHS/United States
- R01 HL075662/HL/NHLBI NIH HHS/United States
- RR20345/RR/NCRR NIH HHS/United States
- P01 HL087123/HL/NHLBI NIH HHS/United States
- R01 AG020255/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous