p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation - PubMed (original) (raw)
. 2008 Oct 23;455(7216):1129-33.
doi: 10.1038/nature07443.
Haoqiang Ying, Haiyan Yan, Alec C Kimmelman, David J Hiller, An-Jou Chen, Samuel R Perry, Giovanni Tonon, Gerald C Chu, Zhihu Ding, Jayne M Stommel, Katherine L Dunn, Ruprecht Wiedemeyer, Mingjian J You, Cameron Brennan, Y Alan Wang, Keith L Ligon, Wing H Wong, Lynda Chin, Ronald A DePinho
Affiliations
- PMID: 18948956
- PMCID: PMC4051433
- DOI: 10.1038/nature07443
p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation
Hongwu Zheng et al. Nature. 2008.
Abstract
Glioblastoma (GBM) is a highly lethal brain tumour presenting as one of two subtypes with distinct clinical histories and molecular profiles. The primary GBM subtype presents acutely as a high-grade disease that typically harbours mutations in EGFR, PTEN and INK4A/ARF (also known as CDKN2A), and the secondary GBM subtype evolves from the slow progression of a low-grade disease that classically possesses PDGF and TP53 events. Here we show that concomitant central nervous system (CNS)-specific deletion of p53 and Pten in the mouse CNS generates a penetrant acute-onset high-grade malignant glioma phenotype with notable clinical, pathological and molecular resemblance to primary GBM in humans. This genetic observation prompted TP53 and PTEN mutational analysis in human primary GBM, demonstrating unexpectedly frequent inactivating mutations of TP53 as well as the expected PTEN mutations. Integrated transcriptomic profiling, in silico promoter analysis and functional studies of murine neural stem cells (NSCs) established that dual, but not singular, inactivation of p53 and Pten promotes an undifferentiated state with high renewal potential and drives increased Myc protein levels and its associated signature. Functional studies validated increased Myc activity as a potent contributor to the impaired differentiation and enhanced renewal of NSCs doubly null for p53 and Pten (p53(-/-) Pten(-/-)) as well as tumour neurospheres (TNSs) derived from this model. Myc also serves to maintain robust tumorigenic potential of p53(-/-) Pten(-/-) TNSs. These murine modelling studies, together with confirmatory transcriptomic/promoter studies in human primary GBM, validate a pathogenetic role of a common tumour suppressor mutation profile in human primary GBM and establish Myc as an important target for cooperative actions of p53 and Pten in the regulation of normal and malignant stem/progenitor cell differentiation, self-renewal and tumorigenic potential.
Figures
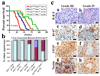
Figure 1. p53 and Pten inactivation cooperate to induce high-grade malignant gliomas
a, Kaplan-Meier tumor-free survival curves for mice of indicated genotypes as a function of weeks. b, Graph shows frequency and grade of gliomas vs. non-CNS malignancies observed in end-stage of indicated mice from a. Asy* indicates neurological asymptomatic hGFAP-Cre;P53lox/lox;Ptenlox/+ mice (n=15) sacrificed for non-CNS malignancies. c, H&E histology and immunohistochemical staining of sections of WHO grade III and grade IV malignant gliomas from hGFAP-Cre;P53lox/lox;Ptenlox/+ mice with antibodies against Ki67, GFAP, and Nestin. Scale bar, 50 µm.
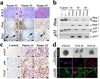
Figure 2. hGFAP-Cre;P53lox/lox;Ptenlox/+ gliomas mirror key features of human malignant gliomas
a, Pten expression is completely extinguished in tumor cells. Sections of three independent malignant gliomas were stained with H&E, and an anti-Pten antibody. Note “N” indicates the adjacent normal regions of the tumor cells; the arrows point to Pten-positive vascular cells embedded in the tumor. b, Wild-type Pten allele is lost in glioma cells. Genomic DNA isolated from liver tissues and brain tumor cells were subjected to PCR-based assays for genotyping Pten and p53 alleles. Note “+” designates the Pten wild-type allele; “_L_” the conditional allele; and “_D_” the inactivated form of the conditional allele after Cre-mediated recombination. c, Immunohistochemical staining of mouse normal brain or glioma sections with antibodies against activated p-AKT, p-S6 kinase, and VEGF. d, TNS lines isolated from independent malignant gliomas were cultured in NSC medium or differentiation medium and immuno-stained for Nestin, GFAP and Tuj1 as indicated. Scale bar, 50 µm.
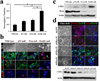
Figure 3. p53 and Pten coordinately regulate c-Myc protein level as well as NSC self-renewal and differentiation
a, The number of neurospheres formed by p53/Pten double-null NSCs in culture is significantly increased as compared to wild-type or singly null NSCs (*P < 0.001; n=3). Values represent mean ± s.d. from three experiments. b, The multi-lineage differentiation potential was impaired in double-null NSCs. Note “WF” designates for white-field; N for Nestin (red); G for GFAP (green); T for Tuj-1 (red); O4 (red); D for DAPI (blue). c, Combined inactivation of p53 and Pten in NSCs stimulates c-Myc protein expression. d, Knockdown of c-Myc expression restores p53/Pten double-null NSCs differentiation capacity. Lower panel, western blot of double-null NSC c-Myc protein expression after infected with indicated lenti-shRNA. Note c-Myc expression in shMyc #2 and #3 infected double-null cells is comparable to that in _p53_-null cells, and shMyc #1 as a control shows no knockdown.
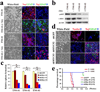
Figure 4. Attenuated c-Myc expression restores hGFAP-Cre;P53lox/lox;Ptenlox/+ TNS differentiation potential and reduces tumorigenic potential
a, Inhibition of the AKT pathway by triciribine induces TNS cell differentiation. Two independent TNS lines were cultured in 1% FBS in the absence or presence of triciribine (5 µM) for 7 days before being subjected to immuno-staining with antibodies against Nestin (red), GFAP (Green) and Tuj1 (red). b, Inhibition of the AKT pathway in TNS cells with triciribine attenuates their cellular c-Myc expression. c, Knockdown of c-Myc expression in TNS cells reduces their self-renewal potential assessed by sphere formation (*P < 0.001, n=3). Values represent mean ± s.d. from three experiments. d, ShRNA-mediated reduction of c-Myc expression in TNS cells sensitizes cells to differentiation stimuli. Cells infected with control and indicated shRNA were incubated with differentiation medium before subjected to indicated lineage marker analysis. e, ShRNA-mediated reduction of c-Myc expression represses TNS tumorigenic potency in orthotopically transplanted SCID mice.
Similar articles
- Pten and p53 converge on c-Myc to control differentiation, self-renewal, and transformation of normal and neoplastic stem cells in glioblastoma.
Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, dePinho RA. Zheng H, et al. Cold Spring Harb Symp Quant Biol. 2008;73:427-37. doi: 10.1101/sqb.2008.73.047. Epub 2009 Jan 15. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19150964 - Gliomagenesis arising from Pten- and Ink4a/Arf-deficient neural progenitor cells is mediated by the p53-Fbxw7/Cdc4 pathway, which controls c-Myc.
Kim HS, Woolard K, Lai C, Bauer PO, Maric D, Song H, Li A, Kotliarova S, Zhang W, Fine HA. Kim HS, et al. Cancer Res. 2012 Nov 15;72(22):6065-75. doi: 10.1158/0008-5472.CAN-12-2594. Epub 2012 Sep 17. Cancer Res. 2012. PMID: 22986743 - Adult Lineage-Restricted CNS Progenitors Specify Distinct Glioblastoma Subtypes.
Alcantara Llaguno SR, Wang Z, Sun D, Chen J, Xu J, Kim E, Hatanpaa KJ, Raisanen JM, Burns DK, Johnson JE, Parada LF. Alcantara Llaguno SR, et al. Cancer Cell. 2015 Oct 12;28(4):429-440. doi: 10.1016/j.ccell.2015.09.007. Cancer Cell. 2015. PMID: 26461091 Free PMC article. - Genetic pathways to primary and secondary glioblastoma.
Ohgaki H, Kleihues P. Ohgaki H, et al. Am J Pathol. 2007 May;170(5):1445-53. doi: 10.2353/ajpath.2007.070011. Am J Pathol. 2007. PMID: 17456751 Free PMC article. Review. - Glioma lateralization: Focus on the anatomical localization and the distribution of molecular alterations (Review).
Cini NT, Pennisi M, Genc S, Spandidos DA, Falzone L, Mitsias PD, Tsatsakis A, Taghizadehghalehjoughi A. Cini NT, et al. Oncol Rep. 2024 Oct;52(4):139. doi: 10.3892/or.2024.8798. Epub 2024 Aug 19. Oncol Rep. 2024. PMID: 39155859 Free PMC article. Review.
Cited by
- The multifaceted role of quaking protein in neuropsychiatric disorders and tumor progression.
Guo Z, Liu B, Wei Y, Wang H, Zhang Q, Hong X. Guo Z, et al. Front Neurosci. 2024 Oct 16;18:1341114. doi: 10.3389/fnins.2024.1341114. eCollection 2024. Front Neurosci. 2024. PMID: 39479357 Free PMC article. Review. - Ruta graveolens L. induces death of glioblastoma cells and neural progenitors, but not of neurons, via ERK 1/2 and AKT activation.
Gentile MT, Ciniglia C, Reccia MG, Volpicelli F, Gatti M, Thellung S, Florio T, Melone MA, Colucci-D'Amato L. Gentile MT, et al. PLoS One. 2015 Mar 18;10(3):e0118864. doi: 10.1371/journal.pone.0118864. eCollection 2015. PLoS One. 2015. PMID: 25785932 Free PMC article. - Tumor suppressive pathways in the control of neurogenesis.
Bartesaghi S, Salomoni P. Bartesaghi S, et al. Cell Mol Life Sci. 2013 Feb;70(4):581-97. doi: 10.1007/s00018-012-1063-9. Epub 2012 Jul 17. Cell Mol Life Sci. 2013. PMID: 22802124 Free PMC article. Review. - Neuroligin 3 Regulates Dendritic Outgrowth by Modulating Akt/mTOR Signaling.
Xu J, Du YL, Xu JW, Hu XG, Gu LF, Li XM, Hu PH, Liao TL, Xia QQ, Sun Q, Shi L, Luo JH, Xia J, Wang Z, Xu J. Xu J, et al. Front Cell Neurosci. 2019 Nov 29;13:518. doi: 10.3389/fncel.2019.00518. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31849609 Free PMC article. - IDP-410: a Novel Therapeutic Peptide that Alters N-MYC Stability and Reduces Angiogenesis and Tumor Progression in Glioblastomas.
Gargini R, Segura-Collar B, Garranzo-Asensio M, Hortigüela R, Iglesias-Hernández P, Lobato-Alonso D, Moreno-Raja M, Esteban-Martin S, Sepúlveda-Sánchez JM, Nevola L, Sánchez-Gómez P. Gargini R, et al. Neurotherapeutics. 2022 Jan;19(1):408-420. doi: 10.1007/s13311-021-01176-6. Epub 2022 Jan 31. Neurotherapeutics. 2022. PMID: 35099769 Free PMC article.
References
- Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat Rev Cancer. 2002;2:616–626. - PubMed
- Furnari FB, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. - PubMed
- Zhuo L, et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA084313/CA/NCI NIH HHS/United States
- R01 CA099041-05/CA/NCI NIH HHS/United States
- R01 CA099041/CA/NCI NIH HHS/United States
- P01 CA095616/CA/NCI NIH HHS/United States
- P01 CA095616-01A19003/CA/NCI NIH HHS/United States
- U01 CA84313/CA/NCI NIH HHS/United States
- R01CA99041/CA/NCI NIH HHS/United States
- 5P01CA95616/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous