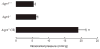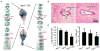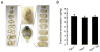Sporadic obstructive hydrocephalus in Aqp4 null mice - PubMed (original) (raw)
Sporadic obstructive hydrocephalus in Aqp4 null mice
Xuechao Feng et al. J Neurosci Res. 2009 Apr.
Abstract
Aquaporin-4 (Aqp4) is a water transport protein expressed in glia and ependymocytes in brain. We report here the unexpected occurrence of severe obstructive hydrocephalus in a random subset of Aqp4 knockout mice. Of 612 Aqp4 knockout mice produced by heterozygote-heterozygote or knockout-knockout breedings, 9.6% of offspring manifested progressive encephalomegaly. Encephalomegaly was never seen in wild-type or Aqp4 heterozygous mice. Examination of the subset encephalomegalic mice revealed marked triventricular hydrocephalus (lateral ventricle size approximately 500 mm(3)), elevated intracranial pressure (19 +/- 3 vs. 6.1 +/- 0.6 mm Hg), and death by age 6 weeks, with a median survival of 28 days. Intraventricular dye injection studies revealed total obstruction of the cerebral aqueduct. Evans blue extravasation studies indicated an intact blood-brain barrier in the hydrocephalic mice. Brain histology revealed reduced ventricular size and ependymocyte disorganization in some nonhydrocephalic Aqp4 null mice. Our studies establish Aqp4 deletion as a predisposing factor for the development of congenital obstructive hydrocephalus in mice. We suggest that AQP4 polymorphisms might also contribute to the development of aqueduct stenosis in humans.
Figures
Fig 1
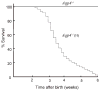
Spontaneous hydrocephalus in Aqp4 null mice. A: External appearance of an Aqp4+/+, a nonhydrocephalic _Aqp4_−/−, and a hydrocephalic _Aqp4_−/− (H) mouse. B: Whole brain (top) and coronal sections (bottom) of brains from a hydrocephalic _Aqp4_−/− (H), a non-hydrocephalic _Aqp4_−/−, and an Aqp4+/+ mouse. Scale in centimeters. [Color figure can be viewed in the online issue, which is available at
.]
Fig 2
Survival of Aqp4 null mice. Curve labeled _Aqp4_−/− represents nonhydrocephalic _Aqp4_−/− mice, and curve labeled _Aqp4_−/−(H) represents mice with visible encephalomegaly by age 3 weeks.
Fig 3
ICP measurements. ICP of hydrocephalic _Aqp4_−/− (H) mice was significantly higher than Aqp4+/+ or nonhydrocephalic Aqp4_−/− mice. Mean ± SD, n = 8 per group. ★_P < 0.001.
Fig 4
Aqueduct stenosis in Aqp4 null hydrocephalic mice. A: Serial coronal sections of the brains from a hydrocephalic _Aqp4_−/− (H) and an Aqp4+/+ mouse after injection of Evans blue dye into a lateral ventricle. Note absence of dye from the fourth ventricle of the _Aqp4_−/− (H) mouse. L, lateral ventricle; 3rd, third ventricle; Aq, aqueduct; 4th, fourth ventricle. B: Hematoxylin–eosin–stained sections through the aqueduct in a hydrocephalic Aqp4_−/− (left) and an Aqp4+/+ (right) mouse. Green arrows show nuclei of ependymal cells arranged as a cuboidal/columnar epithelium. Red arrows indicate region with paucity of ependymal cells. Black arrow shows an abnormal-looking (pyknotic) ependymal cell nucleus forming a multilayered epithelium. C: Analysis of aqueduct shape (as major:minor axis ratio) and size (as area). Mean ± SD, n = 12 per group. ★_P < 0.02. [Color figure can be viewed in the online issue, which is available at
.]
Fig 5
Blood–brain barrier integrity in Aqp4 null hydrocephalic mice. A: Serial coronal brain sections from a hydrocephalic _Aqp4_−/− (H) and an Aqp4+/+ mouse after intravenous Evans blue dye infusion and intravascular washout. B: Amount of extravasated Evans blue dye was not different in the three groups. Mean ± SD, n = 8 per group. [Color figure can be viewed in the online issue, which is available at
.]
Fig 6
Ependymal abnormalities in selected Aqp4 null mice. Magnified view of ependyma in Aqp4+/+ mice and selected nonhydrocephalic _Aqp4_−/− mice where ependymal disorganization was seen. [Color figure can be viewed in the online issue, which is available at
.]
Similar articles
- Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice.
Bloch O, Auguste KI, Manley GT, Verkman AS. Bloch O, et al. J Cereb Blood Flow Metab. 2006 Dec;26(12):1527-37. doi: 10.1038/sj.jcbfm.9600306. Epub 2006 Mar 22. J Cereb Blood Flow Metab. 2006. PMID: 16552421 - Aquaporin-4 maintains ependymal integrity in adult mice.
Li X, Kong H, Wu W, Xiao M, Sun X, Hu G. Li X, et al. Neuroscience. 2009 Aug 4;162(1):67-77. doi: 10.1016/j.neuroscience.2009.04.044. Epub 2009 Apr 22. Neuroscience. 2009. PMID: 19393298 - Impact of aquaporin-4 and CD11c + microglia in the development of ependymal cells in the aqueduct: inferences to hydrocephalus.
Mayo F, González-Vinceiro L, Hiraldo-González L, Rodríguez-Gómez FD, Calle-Castillejo C, Mayo M, Netti V, Ramírez-Lorca R, Echevarría M. Mayo F, et al. Fluids Barriers CNS. 2024 Jul 2;21(1):53. doi: 10.1186/s12987-024-00548-2. Fluids Barriers CNS. 2024. PMID: 38956598 Free PMC article. - AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology.
Saadoun S, Tait MJ, Reza A, Davies DC, Bell BA, Verkman AS, Papadopoulos MC. Saadoun S, et al. Neuroscience. 2009 Jul 7;161(3):764-72. doi: 10.1016/j.neuroscience.2009.03.069. Epub 2009 Apr 5. Neuroscience. 2009. PMID: 19345723 Review. - Hydrocephalus and aquaporins: the role of aquaporin-4.
Filippidis AS, Kalani MY, Rekate HL. Filippidis AS, et al. Acta Neurochir Suppl. 2012;113:55-8. doi: 10.1007/978-3-7091-0923-6_12. Acta Neurochir Suppl. 2012. PMID: 22116424 Review.
Cited by
- Aquaporin 4 deletion exacerbates brain impairments in a mouse model of chronic sleep disruption.
Zhang R, Liu Y, Chen Y, Li Q, Marshall C, Wu T, Hu G, Xiao M. Zhang R, et al. CNS Neurosci Ther. 2020 Feb;26(2):228-239. doi: 10.1111/cns.13194. Epub 2019 Jul 31. CNS Neurosci Ther. 2020. PMID: 31364823 Free PMC article. - The Potential Roles of Aquaporin 4 in Alzheimer's Disease.
Lan YL, Zhao J, Ma T, Li S. Lan YL, et al. Mol Neurobiol. 2016 Oct;53(8):5300-9. doi: 10.1007/s12035-015-9446-1. Epub 2015 Oct 3. Mol Neurobiol. 2016. PMID: 26433375 Review. - Neuromyelitis optica spectrum disorder: Exploring the diverse clinical manifestations and the need for further exploration.
Noori H, Marsool MDM, Gohil KM, Idrees M, Subash T, Alazzeh Z, Prajjwal P, Jain H, Amir O. Noori H, et al. Brain Behav. 2024 Aug;14(8):e3644. doi: 10.1002/brb3.3644. Brain Behav. 2024. PMID: 39135307 Free PMC article. Review. - Leveraging the glymphatic and meningeal lymphatic systems as therapeutic strategies in Alzheimer's disease: an updated overview of nonpharmacological therapies.
Formolo DA, Yu J, Lin K, Tsang HWH, Ou H, Kranz GS, Yau SY. Formolo DA, et al. Mol Neurodegener. 2023 Apr 20;18(1):26. doi: 10.1186/s13024-023-00618-3. Mol Neurodegener. 2023. PMID: 37081555 Free PMC article. Review. - Sex-Dependent Gliovascular Interface Abnormality in the Hippocampus following Postnatal Immune Activation in Mice.
Ardalan M, Chumak T, Quist A, Jabbari Shiadeh SM, Mallard AJ, Rafati AH, Mallard C. Ardalan M, et al. Dev Neurosci. 2022;44(4-5):320-330. doi: 10.1159/000525478. Epub 2022 Jun 15. Dev Neurosci. 2022. PMID: 35705008 Free PMC article.
References
- Bloch O, Papadopoulos MC, Manley GT, Verkman AS. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J Neurochem. 2005;95:254–262. - PubMed
- Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab. 2006;26:1527–1537. - PubMed
- Bruni JE. Ependymal development, proliferation, and functions: a review. Microsc Res Tech. 1998;41:2–13. - PubMed
- Fan Y, Zhang J, Sun XL, Gao L, Zeng XN, Ding JH, Cao C, Niu L, Hu G. Sex- and region-specific alterations of basal amino acid and monoamine metabolism in the brain of aquaporin-4 knockout mice. J Neurosci Res. 2005;82:458–464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases