A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta - PubMed (original) (raw)
A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta
Silvia Balosso et al. Brain. 2008 Dec.
Abstract
Interleukin-1beta (IL-1beta) is overproduced in human and rodent epileptogenic tissue and it exacerbates seizures upon brain application in rodents. Moreover, pharmacological prevention of IL-1beta endogenous synthesis, or IL-1 receptor blockade, mediates powerful anticonvulsive actions indicating a significant role of this cytokine in ictogenesis. The molecular mechanisms of the proconvulsive actions of IL-1beta are not known. We show here that EEG seizures induced by intrahippocampal injection of kainic acid in C57BL6 adult mice were increased by 2-fold on average by pre-exposure to IL-1beta and this effect was blocked by 3-O-methylsphingomyelin (3-O-MS), a selective inhibitor of the ceramide-producing enzyme sphingomyelinase. C2-ceramide, a cell permeable analog of ceramide, mimicked IL-1beta action suggesting that ceramide may be the second messenger of the proconvulsive effect of IL-1beta. The seizure exacerbating effects of either IL-1beta or C2-ceramide were dependent on activation of the Src family of tyrosine kinases since they were prevented by CGP76030, an inhibitor of this enzyme family. The proconvulsive IL-1beta effect was associated with increased Tyr(418) phosphorylation of Src-family of kinases indicative of its activation, and Tyr(1472) phosphorylation of one of its substrate, the NR2B subunit of the N-methyl-d-aspartate receptor, which were prevented by 3-O-MS and CGP76030. Finally, the proconvulsive effect of IL-1beta was blocked by ifenprodil, a selective NR2B receptor antagonist. These results indicate that the proconvulsive actions of IL-1beta depend on the activation of a sphingomyelinase- and Src-family of kinases-dependent pathway in the hippocampus which leads to the phosphorylation of the NR2B subunit, thus highlighting a novel, non-transcriptional mechanism underlying seizure exacerbation in inflammatory conditions.
Figures
Fig. 1
IL-1β and IL-1R1 expression in the mouse hippocampus after kainic acid-induced seizures. Representative photomicrographs of IL-1β (A–B) and IL-1R1 (C–D) immunoreactivity in the CA3 area of the hippocampus, 3 h after seizures induced by intrahippocampal injection of kainic acid (7 ng/0.5 μl; B and D) and in vehicle-injected C57BL6 mice (A, C). IL-1β (A) and IL-1R1 (C) immunostaining was not detectable in control hippocampus. After seizures, IL-1β immunoreactivity is strongly enhanced in GFAP-positive astrocytes (B, yellow signal in inset); IL-1R1 staining was enhanced both in neurons (D, yellow signal in d1) and astrocytes (D, yellow signal in d2). Scale bar: A–D 100 μm; insets, 25 μm.
Fig. 2
Effect of C2-ceramide on seizures. (A) Bargrams represent the mean ± SE (n = 12). Dihydroceramide, the inactive analogue of ceramide, (2 μg/0.5 µl) or C2-ceramide (the cell-permeable analogue of ceramide; 0.25–1.0–2.0 μg/0.5 μl) were injected intrahippocampally, 10 min before kainic acid. Significant increases in seizure parameters were observed at 1 and 2 µg C2-ceramide. *P < 0.05; **P < 0.01 versus dihydroceramide by one way ANOVA followed by Tukey test. (B) Representative EEG tracings of freely moving C57BL6 mice injected unilaterally in the hippocampus with kainic acid ± IL-1β (1 ng/0.5 μl) or C2-ceramide (2 μg/0.5 µl). Treatments or vehicles were given 10 min before kainic acid. (a) Baseline recording before kainic acid injection; arrowheads in (b) and (c) include representative ictal episodes recorded in the EEG during 90 min after kainic acid injection ± IL-1β or C2-ceramide; tracings in (d) depict spiking activity in the EEG after termination of seizures. Either IL-1β or C2-ceramide alone did not induce seizures. RHP and LHP are right and left (injected) hippocampus, respectively.
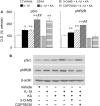
Fig. 3
IL-1β and seizure induced tyrosine phosphorylation of Src-family of kinases and the NR2B subunit of the NMDA receptor: effect of pharmacological treatments. Bargrams show densitometry analysis of the Src kinases and NR2B bands corresponding to their phosphorylated (p) forms (Src-Tyr418; NR2B-Tyr1472) in the various experimental groups, 60 min after the onset of kainate-induced seizures (∼70 min after kainate injection), as assessed by western blot analysis of hippocampal homogenates. Vehicle- or IL-1β alone- treated mice were killed 70 min after the injection. Data (means ± SE, n = 5–7) are optical density (O.D.) values of the relevant bands (as depicted in the representative western blot), divided by the corresponding β-actin value (internal standard). Data are expressed as percentage of values measured in corresponding vehicle-treated mice. IL-1β (1 ng/0.5 μl) was injected alone or 10 min before kainic acid; 3-O-MS (3 µg/0.5 µl) and CGP76030 (65 ng/0.5 µl) were injected 20 min before kainic acid (i.e. 10 min before IL-1β). 3-O-MS or CGP76030 alone did not change protein phosphorylation levels as compared to vehicle-injected mice; total NR2B levels were not changed by the various treatments (not shown). *P < 0.05; **P < 0.01 versus vehicle; ##P < 0.01 versus 3-O-MS+IL-1+KA and versus CGP76030+IL-1+KA (for pNR2B only) by two-way ANOVA followed by Kruskal–Wallis test. Representative western blot bands corresponding to the specific proteins are depicted in B.
Similar articles
- Inhibition of IL-1β Signaling Normalizes NMDA-Dependent Neurotransmission and Reduces Seizure Susceptibility in a Mouse Model of Creutzfeldt-Jakob Disease.
Bertani I, Iori V, Trusel M, Maroso M, Foray C, Mantovani S, Tonini R, Vezzani A, Chiesa R. Bertani I, et al. J Neurosci. 2017 Oct 25;37(43):10278-10289. doi: 10.1523/JNEUROSCI.1301-17.2017. Epub 2017 Sep 18. J Neurosci. 2017. PMID: 28924012 Free PMC article. - IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons.
Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T. Davis CN, et al. J Neurochem. 2006 Sep;98(5):1379-89. doi: 10.1111/j.1471-4159.2006.03951.x. Epub 2006 Jun 12. J Neurochem. 2006. PMID: 16771830 - Interleukin-1-induced interleukin-6 synthesis is mediated by the neutral sphingomyelinase/Src kinase pathway in neurones.
Tsakiri N, Kimber I, Rothwell NJ, Pinteaux E. Tsakiri N, et al. Br J Pharmacol. 2008 Feb;153(4):775-83. doi: 10.1038/sj.bjp.0707610. Epub 2007 Dec 3. Br J Pharmacol. 2008. PMID: 18059318 Free PMC article. - Role of neutral sphingomyelinases in aging and inflammation.
Nikolova-Karakashian M, Karakashian A, Rutkute K. Nikolova-Karakashian M, et al. Subcell Biochem. 2008;49:469-86. doi: 10.1007/978-1-4020-8831-5_18. Subcell Biochem. 2008. PMID: 18751923 Review. - [Interleukin-1 signal transduction in interaction between the nervous and immune systems].
Rybakina EG, Korneva EA. Rybakina EG, et al. Vestn Ross Akad Med Nauk. 2005;(7):3-8. Vestn Ross Akad Med Nauk. 2005. PMID: 16107015 Review. Russian.
Cited by
- Disease modification in epilepsy: from animal models to clinical applications.
Barker-Haliski ML, Friedman D, French JA, White HS. Barker-Haliski ML, et al. Drugs. 2015 May;75(7):749-67. doi: 10.1007/s40265-015-0395-9. Drugs. 2015. PMID: 25925798 Review. - Enhanced Activation of the S1PR2-IL-1β-Src-BDNF-TrkB Pathway Mediates Neuroinflammation in the Hippocampus and Cognitive Impairment in Hyperammonemic Rats.
Sancho-Alonso M, Arenas YM, Izquierdo-Altarejos P, Martinez-Garcia M, Llansola M, Felipo V. Sancho-Alonso M, et al. Int J Mol Sci. 2023 Dec 8;24(24):17251. doi: 10.3390/ijms242417251. Int J Mol Sci. 2023. PMID: 38139078 Free PMC article. - Downregulation of oxidative stress-mediated glial innate immune response suppresses seizures in a fly epilepsy model.
Nukala KM, Lilienthal AJ, Lye SH, Bassuk AG, Chtarbanova S, Manak JR. Nukala KM, et al. Cell Rep. 2023 Jan 31;42(1):112004. doi: 10.1016/j.celrep.2023.112004. Epub 2023 Jan 14. Cell Rep. 2023. PMID: 36641750 Free PMC article. - Hippocampal sclerosis in feline epilepsy.
Wagner E, Rosati M, Molin J, Foitzik U, Wahle AM, Fischer A, Matiasek LA, Reese S, Flegel T, Matiasek K. Wagner E, et al. Brain Pathol. 2014 Nov;24(6):607-19. doi: 10.1111/bpa.12147. Epub 2014 Jun 24. Brain Pathol. 2014. PMID: 24698012 Free PMC article. - "For whom the bell tolls": blockade of toll-like receptors may regulate seizure occurrence.
Barnes GN. Barnes GN. Epilepsy Curr. 2010 Nov;10(6):164-5. doi: 10.1111/j.1535-7511.2010.01389.x. Epilepsy Curr. 2010. PMID: 21157547 Free PMC article. No abstract available.
References
- Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–42. - PubMed
- Aronica E, Boer K, van Vliet EA, Redeker S, Baayen JC, Spliet WG, et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol Dis. 2007;26:497–511. - PubMed
- Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, et al. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol. 2005;57:804–12. - PubMed
- Bock J, Szabo I, Gamper N, Adams C, Gulbins E. Ceramide inhibits the potassium channel Kv1.3 by the formation of membrane platforms. Biochem Biophys Res Commun. 2003;305:890–7. - PubMed
- Chenard BL, Menniti FS. Antagonists selective for NMDA receptors containing the NR2B subunit. Curr Pharm Des. 1999;5:381–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous