Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy - PubMed (original) (raw)
Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy
Jing Chen et al. Invest Ophthalmol Vis Sci. 2009 Mar.
Abstract
Purpose: Erythropoietin (EPO), an oxygen-regulated hormone stimulating erythrocyte production, was recently found to be critical for retinal angiogenesis. EPO mRNA expression levels in retina are highly elevated during the hypoxia-induced proliferation phase of retinopathy. The authors investigated the inhibition of retinal EPO mRNA expression with RNA interference as a potential strategy to suppress retinal neovascularization and to prevent proliferative retinopathy.
Methods: The authors used a mouse model of oxygen-induced retinopathy. Retinal EPO and Epo receptor (EpoR) expression during retinopathy development were quantified with real-time RT-PCR in whole retina and on laser-captured retinal vessels and neuronal layers. Retinal hypoxia was assessed with an oxygen-sensitive hypoxyprobe. A small interference RNA (siRNA) targeting EPO or control negative siRNA was injected intravitreally at postnatal (P) day 12, P14, and P15 during the hypoxic phase, and the effect on neovascularization was evaluated in retinal flatmounts at P17.
Results: Retinal EPO mRNA expression in total retina was suppressed during the initial phase of vessel loss in retinopathy and was significantly elevated during the hypoxia-induced proliferative phase in all three neuronal layers in the retina, corresponding to an increased level of retinal hypoxia. EpoR mRNA expression levels also increased during the second neovascular phase, specifically in hypoxia-induced neovascular vessels. Intravitreous injection of EPO siRNA effectively inhibited approximately 60% of retinal EPO mRNA expression and significantly suppressed retinal neovascularization by approximately 40%.
Conclusions: Inhibiting EPO mRNA expression with siRNA is effective in suppressing retinal neovascularization, suggesting EPO siRNA is a potentially useful pharmaceutical intervention for treating proliferative retinopathy.
Figures
Figure 1
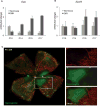
EPO and EpoR expression during oxygen-induced retinopathy. Real-time PCR quantification of (A) EPO and (B) EpoR mRNA expression in age-matched mouse retinas under normoxia or oxygen treatment. Copy number of mRNA/million copies of cyclophilin A control mRNA at P10, P14, P15, and P17 (n = 6 per group). (C) Representative retinal whole mounts from P15 oxygen-treated mouse showing deposits of hypoxyprobe at site of tissue hypoxia during OIR. Original magnification, ×5. A portion of the image was enlarged (right, white frame). Vessels were visualized with isolection B4 staining (red). Hypoxyprobe staining (green) is evident in central avascular retina but not in peripheral vascularized retina.
Figure 2
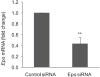
EPO siRNA effectively inhibited EPO expression. Fellow eyes of oxygen-treated mice were injected at P12 with negative control siRNA or EPO siRNA. At P14, retinas were isolated, and expression of EPO mRNA was quantified with quantitative real-time PCR (n = 5; **P ≤ 0.005, comparing matched fellow eyes). Retinal EPO levels after control or EPO siRNA treatment ranged from 200 to 500 copies per 106 cyclophilin A control but are compared with control to preserve the fellow eye comparisons.
Figure 3
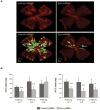
EPO siRNA suppresses vaso-obliteration and neovascularization in retinopathy. Fellow eyes of oxygen-treated mice were intravitreally injected with negative control siRNA or EPO siRNA at P12, P14, and P15. (A) Representative retina whole mount at P17 showing vaso-obliteration (VO; green) and neovascularization (NV; yellow). Vessels were visualized with isolectin B4 staining (red). Original magnification, ×5. (B) Areas of VO and NV were quantified as percentages of total retina area. P12, n = 11; P14, n = 5; P15, n = 9. ***P ≤ 0.001; *P ≤ 0.05.
Figure 4
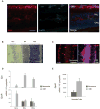
Localization of EPO and EpoR in oxygen-induced retinopathy. (A) Representative retinal cross-sections from P17 oxygen-treated mouse immunolabeled with EPO antibody (red), isolectin B4 to visualize vessels (green), and DAPI (blue). (B) Quantitative localization of EPO and EpoR expression in response to oxygen-induced retinopathy with LCM. Representative cross-section from P17 normoxia retina stained with hematoxylin and eosin for LCM. Three neuronal layers were laser captured from normoxia and oxygen-treated P17 retinas: ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL). (C) Cross-sections of normoxia control and OIR retinas stained for vessels with isolectin B4 (red). Normoxic vessels and angiogenic tufts were captured by LCM. (D) Quantification of EPO and EpoR mRNA expression in microdissected retinal neuronal layers with RT-PCR. Samples were normalized to 106 copies of cyclophilin. (E) Quantification of EpoR mRNA expression in vessels from normoxic mouse and angiogenic tufts from oxygen- treated mouse at P17. EPO expression in vessels and tufts was negligible (data not shown). Scale bar, 50 _μ_m.
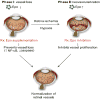
Figure 5
Schematic representation of EPO control of blood vessel development during retinopathy. Opposite approaches of EPO intervention are required during the two phases of retinopathy to normalize retinal vessels. During the first phase of retinopathy, vascular growth ceases and normal vessels degenerate. High oxygen exposure (as occurs in animal models and in some premature infants) and compromised kidney function (as in some patients with diabetes) suppress EPO, further contributing to inhibition of vessel growth. Supplementation of EPO during this phase may prevent vessel loss through systemic recruitment of bone marrow–derived proangiogenic stem cells into the retina and through local mechanisms such as prosurvival (NF-_κ_B), antiapoptosis (caspase) pathways. During the second neovascular phase of retinopathy, hypoxia increases EPO expression, which may precipitate vessel proliferation. Inhibition of EPO (e.g., with siRNA) during this phase is likely beneficial.
Similar articles
- Suppression of retinal neovascularization by small-interference RNA targeting erythropoietin.
Xiong SQ, Xia XB, Xu HZ, Jiang J. Xiong SQ, et al. Ophthalmologica. 2009;223(5):306-12. doi: 10.1159/000215825. Epub 2009 Apr 30. Ophthalmologica. 2009. PMID: 19407475 - Expression profile and regulation of telomerase reverse transcriptase on oxygen-induced retinal neovascularization.
Min X, Zhou Q, Dong X, Wang Y, Xie L. Min X, et al. Curr Eye Res. 2011 Feb;36(2):135-42. doi: 10.3109/02713683.2010.525679. Epub 2010 Dec 15. Curr Eye Res. 2011. PMID: 21158588 Retracted. - Soluble forms of EphrinB2 and EphB4 reduce retinal neovascularization in a model of proliferative retinopathy.
Zamora DO, Davies MH, Planck SR, Rosenbaum JT, Powers MR. Zamora DO, et al. Invest Ophthalmol Vis Sci. 2005 Jun;46(6):2175-82. doi: 10.1167/iovs.04-0983. Invest Ophthalmol Vis Sci. 2005. PMID: 15914639 - The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model.
Hartnett ME. Hartnett ME. Doc Ophthalmol. 2010 Feb;120(1):25-39. doi: 10.1007/s10633-009-9181-x. Epub 2009 Jul 29. Doc Ophthalmol. 2010. PMID: 19639355 Free PMC article. Review. - The significance of neuronal and glial cell changes in the rat retina during oxygen-induced retinopathy.
Fletcher EL, Downie LE, Hatzopoulos K, Vessey KA, Ward MM, Chow CL, Pianta MJ, Vingrys AJ, Kalloniatis M, Wilkinson-Berka JL. Fletcher EL, et al. Doc Ophthalmol. 2010 Feb;120(1):67-86. doi: 10.1007/s10633-009-9193-6. Epub 2009 Sep 8. Doc Ophthalmol. 2010. PMID: 19763649 Review.
Cited by
- Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice.
Ohlmann A, Seitz R, Braunger B, Seitz D, Bösl MR, Tamm ER. Ohlmann A, et al. J Neurosci. 2010 Jan 6;30(1):183-93. doi: 10.1523/JNEUROSCI.3210-09.2010. J Neurosci. 2010. PMID: 20053900 Free PMC article. - Ghrelin modulates physiologic and pathologic retinal angiogenesis through GHSR-1a.
Zaniolo K, Sapieha P, Shao Z, Stahl A, Zhu T, Tremblay S, Picard E, Madaan A, Blais M, Lachapelle P, Mancini J, Hardy P, Smith LE, Ong H, Chemtob S. Zaniolo K, et al. Invest Ophthalmol Vis Sci. 2011 Jul 23;52(8):5376-86. doi: 10.1167/iovs.10-7152. Invest Ophthalmol Vis Sci. 2011. PMID: 21642627 Free PMC article. - Retinopathy of prematurity: current concepts in molecular pathogenesis.
Heidary G, Vanderveen D, Smith LE. Heidary G, et al. Semin Ophthalmol. 2009 Mar-Apr;24(2):77-81. doi: 10.1080/08820530902800314. Semin Ophthalmol. 2009. PMID: 19373690 Free PMC article. Review.
References
- Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. - PubMed
- Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438(7070):960–966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY14811/EY/NEI NIH HHS/United States
- EY017017/EY/NEI NIH HHS/United States
- F32 EY017789/EY/NEI NIH HHS/United States
- R01 EY008670-14/EY/NEI NIH HHS/United States
- R01 EY017017/EY/NEI NIH HHS/United States
- R21 EY014811-01/EY/NEI NIH HHS/United States
- 1 F32EY017789-01/EY/NEI NIH HHS/United States
- P01 HD18655/HD/NICHD NIH HHS/United States
- F32 EY017789-01A1/EY/NEI NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- 5 T32 EY07145/EY/NEI NIH HHS/United States
- Y008670/PHS HHS/United States
- R01 EY022084/EY/NEI NIH HHS/United States
- T32 EY007145-05/EY/NEI NIH HHS/United States
- R21 EY014811/EY/NEI NIH HHS/United States
- R01 EY008670/EY/NEI NIH HHS/United States
- R01 EY017017-01/EY/NEI NIH HHS/United States
- T32 EY007145/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials