A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis - PubMed (original) (raw)
Clinical Trial
A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis
John C McAuliffe et al. Ann Surg Oncol. 2009 Apr.
Abstract
Gastrointestinal stromal tumor (GIST) is the most common sarcoma arising in the gastrointestinal (GI) tract. Imatinib mesylate (imatinib) is efficacious in treating advanced and metastatic GIST. Patients undergoing resection of GIST realize a highly variable median disease-free survival (DFS). In the absence of prospective data, we conducted a randomized, phase II study to assess the safety and efficacy of preoperative and postoperative imatinib for the treatment of GIST. Nineteen GIST patients undergoing surgical resection were randomized to receive 3, 5, or 7 days of preoperative imatinib (600 mg daily). Patients received postoperative imatinib for 2 years. Perioperative adverse events were compared with those in an imatinib-naïve historical control. The efficacy of imatinib was assessed by (18)fluorodeoxyglucose positron emission tomography ((18)FDG-PET), dynamic computed tomography (dCT), terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, and DFS. Imatinib did not affect surgical morbidity as compared with an imatinib-naïve cohort (p >/= 0.1). Most patients responded to preoperative imatinib by (18)FDG-PET and dCT (69% and 71%, respectively). Tumor cell apoptosis increased by an average of 12% (range 0-33%) and correlated with the duration of preoperative imatinib (p = 0.04). Median DFS of patients treated with surgery and imatinib was 46 months (range 10-46 months). Tumor size was a predictor of recurrence after postoperative imatinib (p = 0.02). Imatinib appears to be safe and may be considered for patients undergoing surgical resection of their GIST. Radiographic response and tumor cell apoptosis occur within the first week of imatinib therapy.
Figures
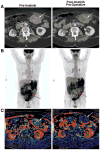
Fig. 1
Representative radiographic and functional imaging of GIST (arrows). (a) contrast-enhanced CT before and after preoperative imatinib. Some hypodensity within the tumor can be appreciated in presurgical scans compared with baseline scans. (b) 18FDG-PET before and after preoperative imatinib. Black indicates sites of 18FDG accumulation. Complete abrogation of avid disease can be appreciated in this representative patient in latter scans. (c) dCT blood flow reconstruction before and after preoperative imatinib. Red indicates blood flow similar to the abdominal aorta; other colors indicate blood flow less than the abdominal aorta, with blue and black representing the least blood flow. A decrease in the amount of red within the tumor in latter scans compared to pre-imatinib scans indicates a decrease in tumor blood flow in response to imatinib
Fig. 2
Histologic evaluation of tumor tissue and TUNEL assay. Matched hematoxylin and eosin (H&E) and immunofluorescence of a representative frozen biopsy and surgical specimen from a single patient treated for 5 days with preoperative imatinib. Hypercellular, hyperchromic tissue can be appreciated in both the biopsy and surgical specimens. TUNEL assay indicates that tumor cells underwent apoptosis after 5 days of imatinib: blue, nuclei stained with DAPI; green, fluorescein isothiocyanate (FITC) tag of positive TUNEL reaction; merge, overlay of blue and green indicating nuclei undergoing apoptosis
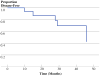
Fig. 3
Disease-free survival. Kaplan–Meier plot of DFS versus time (months) since recruitment to trial. Median DFS = 46 months. Tick marks = patients censored
Comment in
- Nanoneoadjuvant therapy of gastrointestinal stromal tumor (GIST).
DeMatteo RP. DeMatteo RP. Ann Surg Oncol. 2009 Apr;16(4):799-800. doi: 10.1245/s10434-009-0316-9. Epub 2009 Jan 24. Ann Surg Oncol. 2009. PMID: 19169754 No abstract available.
Similar articles
- Early effects of imatinib mesylate on the expression of insulin-like growth factor binding protein-3 and positron emission tomography in patients with gastrointestinal stromal tumor.
Trent JC, Ramdas L, Dupart J, Hunt K, Macapinlac H, Taylor E, Hu L, Salvado A, Abbruzzese JL, Pollock R, Benjamin RS, Zhang W. Trent JC, et al. Cancer. 2006 Oct 15;107(8):1898-908. doi: 10.1002/cncr.22214. Cancer. 2006. PMID: 16986125 - Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate.
Rutkowski P, Nowecki Z, Nyckowski P, Dziewirski W, Grzesiakowska U, Nasierowska-Guttmejer A, Krawczyk M, Ruka W. Rutkowski P, et al. J Surg Oncol. 2006 Mar 15;93(4):304-11. doi: 10.1002/jso.20466. J Surg Oncol. 2006. PMID: 16496358 - [18F-FDG PET/CT in response evaluation of gastrointestinal stromal tumours treated with imatinib].
Banzo I, Quirce R, Martínez-Rodríguez I, Jiménez-Bonilla JF, Sainz-Esteban A, Barragán J, Portilla-Quattrociocchi H, Carril JM. Banzo I, et al. Rev Esp Med Nucl. 2008 May-Jun;27(3):168-75. Rev Esp Med Nucl. 2008. PMID: 18570858 Clinical Trial. Spanish. - [Indications for pre- and postoperative treatment with imatinib for gastrointestinal stromal tumors].
Heger U, Weitz J, Lordick F. Heger U, et al. Chirurg. 2008 Jul;79(7):630-7. doi: 10.1007/s00104-008-1526-6. Chirurg. 2008. PMID: 18548219 Review. German. - Imatinib rechallenge in patients with advanced gastrointestinal stromal tumors.
Blay JY, Pérol D, Le Cesne A. Blay JY, et al. Ann Oncol. 2012 Jul;23(7):1659-65. doi: 10.1093/annonc/mdr622. Epub 2012 Feb 21. Ann Oncol. 2012. PMID: 22357253 Review.
Cited by
- A gist of gastrointestinal stromal tumors: A review.
Rammohan A, Sathyanesan J, Rajendran K, Pitchaimuthu A, Perumal SK, Srinivasan U, Ramasamy R, Palaniappan R, Govindan M. Rammohan A, et al. World J Gastrointest Oncol. 2013 Jun 15;5(6):102-12. doi: 10.4251/wjgo.v5.i6.102. World J Gastrointest Oncol. 2013. PMID: 23847717 Free PMC article. - Esophageal gastrointestinal stromal tumor: report of 7 patients.
Shinagare AB, Zukotynski KA, Krajewski KM, Jagannathan JP, Butrynski J, Hornick JL, Ramaiya NH. Shinagare AB, et al. Cancer Imaging. 2012 Apr 27;12(1):100-8. doi: 10.1102/1470-7330.2012.0017. Cancer Imaging. 2012. PMID: 22542728 Free PMC article. - Efficacy and economic value of adjuvant imatinib for gastrointestinal stromal tumors.
Rutkowski P, Gronchi A. Rutkowski P, et al. Oncologist. 2013 Jun;18(6):689-96. doi: 10.1634/theoncologist.2012-0474. Epub 2013 May 24. Oncologist. 2013. PMID: 23709752 Free PMC article. - Accomplishments in 2008 in the management of gastrointestinal stromal tumors.
Renouf D, Blay JY, Blanke C. Renouf D, et al. Gastrointest Cancer Res. 2009 Sep;3(5 Supplement 2):S67-72. Gastrointest Cancer Res. 2009. PMID: 20011569 Free PMC article. - Pretreatment Tumor DNA Sequencing of KIT and PDGFRA in Endosonography-Guided Biopsies Optimizes the Preoperative Management of Gastrointestinal Stromal Tumors.
Hedenström P, Andersson C, Sjövall H, Enlund F, Nilsson O, Nilsson B, Sadik R. Hedenström P, et al. Mol Diagn Ther. 2020 Apr;24(2):201-214. doi: 10.1007/s40291-020-00451-0. Mol Diagn Ther. 2020. PMID: 32124386 Free PMC article. Clinical Trial.
References
- Steinert DM, McAuliffe JC, Trent JC. Imatinib mesylate in the treatment of gastrointestinal stromal tumour. Expert Opin Pharmacother. 2005;6:105–13. - PubMed
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. - PubMed
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. - PubMed
- Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1:493–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA-16672/CA/NCI NIH HHS/United States
- TL1 RR 024147/RR/NCRR NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- K12 CA 090891/CA/NCI NIH HHS/United States
- K23 CA109060/CA/NCI NIH HHS/United States
- K12 CA090891/CA/NCI NIH HHS/United States
- R01 CA083932/CA/NCI NIH HHS/United States
- TL1 RR024147/RR/NCRR NIH HHS/United States
- 1K23 CA 109060-02/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources