Differential expression of epigenetic modulators during human embryonic stem cell differentiation - PubMed (original) (raw)
Differential expression of epigenetic modulators during human embryonic stem cell differentiation
Sharla M O Phipps et al. Mol Biotechnol. 2009 Mar.
Abstract
Although the progression of aging and the diseases associated with it are extensively studied, little is known about the initiation of the aging process. Telomerase is down-regulated early in embryonic differentiation, thereby contributing to telomeric attrition and aging. The mechanisms underlying this inhibition remain elusive, but epigenetic studies in differentiating human embryonic stem (hES) cells could give clues about how and when DNA methylation and histone deacetylation work together to contribute to the inactivation of hTERT, the catalytic subunit of telomerase, at the onset of the aging process. We have confirmed the differentiation status of cultured hES colonies with morphological assessment and immunohistochemical stainings for pluripotent stem cells. In hES cells with varying degrees of differentiation, we have shown a stronger association between hES differentiation and expression of the epigenetic regulators DNMT3A and DNMT3B than between genetic modulators of differentiation such as c-MYC. We also propose a new model system for analyses of stem cell regions, which are differentially down-regulating the expression of hTERT and the actions of epigenetic modulators such as the DNMTs and histone methyltransferases.
Figures
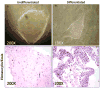
Fig. 1
Phase contrast photography and hematoxylin and eosin staining of differentiating hES colonies. Colonies were induced to differentiate by growing them without routine passaging for an additional 2 weeks after the mouse feeders expired (a total of approximately 1 month). Phase contrast photographs were taken at 200× and 100× of undifferentiated and differentiating stem cell colonies, respectively. Hematoxylin and eosin staining of harvested hES colony sections were taken at 200× magnification
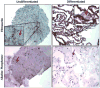
Fig. 2
Molecular evidence of hES differentiation status. Immunohistochemistry staining for the glycoprotein FN that is only expressed after cells have committed to differentiation. Confirmation of the differentiation status of hES colonies with immunohistochemical staining for the marker AP. Photographs were taken at 200× magnification. Arrows indicate examples of staining for FN or AP. Insets are provided for close-up viewing of stains
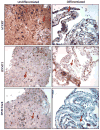
Fig. 3
hTERT and DNMT immunohistochemistry. Photographs taken at 200× illustrate the expression of the catalytic subunit of telomerase, hTERT. hTERT is only visible in differentiated sections in the mouse-feeder layer because of cross-reactivity of the antibody. The undifferentiated section displays abundant hTERT staining throughout the entire colony, both periphery and core. Staining for DNMT1 displays a decrease but not total loss of expression, whereas staining for DNMT3a/3b does indicate a drastic loss of expression. Arrows indicate nuclear staining examples
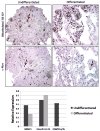
Fig. 4
Dimethylated histone 3 lysine 9 (H3-K9) and c-Myc immunohistochemistry. Photographs were taken at 200× magnification. Staining for dimethyl-H3-K9 illustrates a clear increase in the level of histone methylation in differentiated cells. There is positive c-MYC staining in the undifferentiated section, but not in the differentiated section. Arrows indicate staining examples of nuclei. The graphical representation at the bottom shows the relative expression of the three epigenetic proteins, DNMT1, DNMT3a/3b, and dimethyl-H3-K9
Similar articles
- TERT Promoter Methylation Is Oxygen-Sensitive and Regulates Telomerase Activity.
Dogan F, Forsyth NR. Dogan F, et al. Biomolecules. 2024 Jan 19;14(1):131. doi: 10.3390/biom14010131. Biomolecules. 2024. PMID: 38275760 Free PMC article. - Aza-induced cardiomyocyte differentiation of P19 EC-cells by epigenetic co-regulation and ERK signaling.
Abbey D, Seshagiri PB. Abbey D, et al. Gene. 2013 Sep 10;526(2):364-73. doi: 10.1016/j.gene.2013.05.044. Epub 2013 Jun 7. Gene. 2013. PMID: 23747406 - Epigenetic mechanisms regulate placental c-myc and hTERT in normal and pathological pregnancies; c-myc as a novel fetal DNA epigenetic marker for pre-eclampsia.
Rahat B, Hamid A, Ahmad Najar R, Bagga R, Kaur J. Rahat B, et al. Mol Hum Reprod. 2014 Oct;20(10):1026-40. doi: 10.1093/molehr/gau053. Epub 2014 Jul 14. Mol Hum Reprod. 2014. PMID: 25024139 - Epigenetics in embryonic stem cells: regulation of pluripotency and differentiation.
Atkinson S, Armstrong L. Atkinson S, et al. Cell Tissue Res. 2008 Jan;331(1):23-9. doi: 10.1007/s00441-007-0536-x. Epub 2007 Nov 15. Cell Tissue Res. 2008. PMID: 18004593 Review. - The role of mammalian DNA methyltransferases in the regulation of gene expression.
Turek-Plewa J, Jagodziński PP. Turek-Plewa J, et al. Cell Mol Biol Lett. 2005;10(4):631-47. Cell Mol Biol Lett. 2005. PMID: 16341272 Review.
Cited by
- TERT Promoter Methylation Is Oxygen-Sensitive and Regulates Telomerase Activity.
Dogan F, Forsyth NR. Dogan F, et al. Biomolecules. 2024 Jan 19;14(1):131. doi: 10.3390/biom14010131. Biomolecules. 2024. PMID: 38275760 Free PMC article. - Telomerase Regulation: A Role for Epigenetics.
Dogan F, Forsyth NR. Dogan F, et al. Cancers (Basel). 2021 Mar 10;13(6):1213. doi: 10.3390/cancers13061213. Cancers (Basel). 2021. PMID: 33802026 Free PMC article. Review. - Promoter hypermethylation as a mechanism for Lamin A/C silencing in a subset of neuroblastoma cells.
Rauschert I, Aldunate F, Preussner J, Arocena-Sutz M, Peraza V, Looso M, Benech JC, Agrelo R. Rauschert I, et al. PLoS One. 2017 Apr 19;12(4):e0175953. doi: 10.1371/journal.pone.0175953. eCollection 2017. PLoS One. 2017. PMID: 28422997 Free PMC article. - Functional genomics of 5- to 8-cell stage human embryos by blastomere single-cell cDNA analysis.
Galán A, Montaner D, Póo ME, Valbuena D, Ruiz V, Aguilar C, Dopazo J, Simón C. Galán A, et al. PLoS One. 2010 Oct 26;5(10):e13615. doi: 10.1371/journal.pone.0013615. PLoS One. 2010. PMID: 21049019 Free PMC article.
References
- Shamblott MJ, Axelman J, Littlefield JW, et al. Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:113–118. doi: 10.1073/pnas.021537998. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources