Mouse model of liver ischemia and reperfusion injury: method for studying reactive oxygen and nitrogen metabolites in vivo - PubMed (original) (raw)
Mouse model of liver ischemia and reperfusion injury: method for studying reactive oxygen and nitrogen metabolites in vivo
Yuta Abe et al. Free Radic Biol Med. 2009.
Abstract
The mouse model of liver ischemia and reperfusion injury has proven to be valuable for our understanding of the role that reactive oxygen and nitrogen metabolites play in postischemic tissue injury. This methods paper provides a detailed protocol for inducing partial liver ischemia followed by reperfusion. Liver ischemia is induced in anesthetized mice by cross-clamping the hepatic artery and portal vein for varying lengths of time, resulting in deprivation of blood flow to approximately 70% of the liver. Restoration of blood flow to the ischemic lobes enhances superoxide production concomitant with a rapid and marked decrease in the bioavailability of nitric oxide, resulting in alterations in the redox state of the liver in favor of a more oxidative environment. This hepatocellular oxidative stress induces the activation of oxidant-sensitive transcription factors followed by the upregulation of proinflammatory cytokines and mediators that ultimately lead to liver injury. This model can be induced in any strain or sex of mouse and requires 1-2 months of practice to become proficient in the surgery and animal manipulation. The roles of various reactive metabolites of oxygen and nitrogen may be evaluated using genetically engineered mice as well as selective molecular, cellular, and/or pharmacological agents.
Figures
Figure 1
Drawing depicting a ventral view of the normal mouse liver in which the left lateral and median lobes have been reflected back to expose the portal vein (blue), hepatic artery (red) and common bile duct (green). Cross clamping the portal vein and hepatic artery induces ischemia to the left lateral and median lobes of the liver.
Figure 2
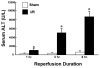
Serum alanine aminotransferase (ALT) levels following 90 minutes of ischemia and varying periods of reperfusion. Sham operated animals underwent identical surgical manipulations without clamp placement. * p< 0.05 for I/R (black bar) versus time-matched sham-operated controls (white bar). Data derived from reference .
Figure 3
Histopathology of livers subjected to sham surgery or 90 minutes of ischemia and 6 hours of reperfusion. A. Sham operated control liver. B. Liver subjected to 90 minutes of ischemia and 6 hours of reperfusion. Note the absence of PMN infiltration but the presence of hepatocellular necrosis, vacuolization and pyknotic nuclei.
Figure 4
Electromobility shift assay (EMSA) demonstrating activation and nuclear localization of the p50/p65 heterodimeric transcription factor NFkB following 90 minutes of ischemia and varying times of reperfusion.
Figure 5
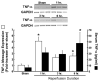
Liver message and serum TNF-α levels following 90 minutes of ischemia and varying periods of reperfusion. * p< 0.05 vs. sham operated control.
Figure 6
Effect of a monoclonal antibody to mouse TNF-α (200 ug/mouse) on serum ALT levels following 90 minutes of ischemia and varying periods of reperfusion. * p< 0.05 for antibody treated versus vehicle-treated control. Data derived from reference .
Figure 7
Effect of polycationic SOD2/3 on serum ALT levels following 90 minutes of ischemia and varying times of reperfusion. * p<0.05 versus sham operated control. Data derived from reference .
Figure 8
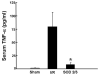
Effect of polycationic SOD2/3 on serum TNF-α protein expression following 90 minutes of ischemia and 6 hours of reperfusion. * p<0.05 versus sham operated control. Data derived from reference .
Figure 9
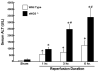
Exacerbation of liver I/R injury in eNOS deficient (eNOS-/-) mice following 45 minutes of ischemia and varying times of reperfusion. *p<0.05 vs. sham; #p<0.05 vs. wild type. Data derived from reference .
References
- Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. - PubMed
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. - PubMed
- Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. - PubMed
- Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. - PubMed
- Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources