Molecular mechanisms of alpha-synuclein neurodegeneration - PubMed (original) (raw)
Review
Molecular mechanisms of alpha-synuclein neurodegeneration
Elisa A Waxman et al. Biochim Biophys Acta. 2009 Jul.
Abstract
alpha-Synuclein is an abundant highly charged protein that is normally predominantly localized around synaptic vesicles in presynaptic terminals. Although the function of this protein is still ill-defined, genetic studies have demonstrated that point mutations or genetic alteration (duplications or triplications) that increase the number of copies of the alpha-synuclein (SCNA) gene can cause Parkinson's disease or the related disorder dementia with Lewy bodies. alpha-Synuclein can aberrantly polymerize into fibrils with typical amyloid properties, and these fibrils are the major component of many types of pathological inclusions, including Lewy bodies, which are associated with neurodegenerative diseases, such as Parkinson's disease. Although there is substantial evidence supporting the toxic nature of alpha-synuclein inclusions, other modes of toxicity such as oligomers have been proposed. In this review, some of the evidence for the different mechanisms of alpha-synuclein toxicity is presented and discussed.
Figures
Figure 1. Amino acid sequence and regions of α-synuclein
α-Syn is composed of: 1) an amino-terminal domain (black) containing several imperfect KTKEGV motifs (blue underline); 2) a hydrophobic center (purple) termed non-amyloid component (NAC); and 3) a negatively charged carboxy-terminus (green). Three familial mutations in α-syn (red) have been identified in patients with PD.
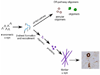
Figure 2. Formation of α-synuclein polymeric intermediates and fibrils
α-Syn in native, monomeric form is mostly unstructured. Under certain conditions α-syn can undergo structural changes, resulting in β-pleated sheet formation. This form of α-syn can take two pathways, one which is off of the fibrillar pathway, and the other which will eventually form mature fibrils. The off-fibril pathway can result in the formation of annular or other forms of oligomers that will never develop into mature fibrils. The fibrillar pathway undergoes intermediate stages, which include protofibrils, before maturing into long strands and becoming LBs or LNs.
Similar articles
- Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder?
Olanow CW, Brundin P. Olanow CW, et al. Mov Disord. 2013 Jan;28(1):31-40. doi: 10.1002/mds.25373. Mov Disord. 2013. PMID: 23390095 Review. - How can rAAV-α-synuclein and the fibril α-synuclein models advance our understanding of Parkinson's disease?
Volpicelli-Daley LA, Kirik D, Stoyka LE, Standaert DG, Harms AS. Volpicelli-Daley LA, et al. J Neurochem. 2016 Oct;139 Suppl 1(Suppl 1):131-155. doi: 10.1111/jnc.13627. Epub 2016 May 4. J Neurochem. 2016. PMID: 27018978 Free PMC article. Review. - Alteration of Structure and Aggregation of α-Synuclein by Familial Parkinson's Disease Associated Mutations.
Sahay S, Ghosh D, Singh PK, Maji SK. Sahay S, et al. Curr Protein Pept Sci. 2017;18(7):656-676. doi: 10.2174/1389203717666160314151706. Curr Protein Pept Sci. 2017. PMID: 26972727 Review. - Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery.
Lee VM, Trojanowski JQ. Lee VM, et al. Neuron. 2006 Oct 5;52(1):33-8. doi: 10.1016/j.neuron.2006.09.026. Neuron. 2006. PMID: 17015225 Review. - Threonine 53 in alpha-synuclein is conserved in long-living non-primate animals.
Larsen K, Hedegaard C, Bertelsen MF, Bendixen C. Larsen K, et al. Biochem Biophys Res Commun. 2009 Sep 25;387(3):602-5. doi: 10.1016/j.bbrc.2009.07.070. Epub 2009 Jul 18. Biochem Biophys Res Commun. 2009. PMID: 19619507
Cited by
- From the Cover: Arsenic Induces Accumulation of α-Synuclein: Implications for Synucleinopathies and Neurodegeneration.
Cholanians AB, Phan AV, Ditzel EJ, Camenisch TD, Lau SS, Monks TJ. Cholanians AB, et al. Toxicol Sci. 2016 Oct;153(2):271-81. doi: 10.1093/toxsci/kfw117. Epub 2016 Jul 13. Toxicol Sci. 2016. PMID: 27413109 Free PMC article. - Mechanisms of action of brain insulin against neurodegenerative diseases.
Ramalingam M, Kim SJ. Ramalingam M, et al. J Neural Transm (Vienna). 2014 Jun;121(6):611-26. doi: 10.1007/s00702-013-1147-1. Epub 2014 Jan 9. J Neural Transm (Vienna). 2014. PMID: 24398779 Review. - Comparison of the in vivo induction and transmission of α-synuclein pathology by mutant α-synuclein fibril seeds in transgenic mice.
Rutherford NJ, Dhillon JS, Riffe CJ, Howard JK, Brooks M, Giasson BI. Rutherford NJ, et al. Hum Mol Genet. 2017 Dec 15;26(24):4906-4915. doi: 10.1093/hmg/ddx371. Hum Mol Genet. 2017. PMID: 29036344 Free PMC article. - Mitochondrial mutations: newly discovered players in neuronal degeneration.
Lax NZ, Turnbull DM, Reeve AK. Lax NZ, et al. Neuroscientist. 2011 Dec;17(6):645-58. doi: 10.1177/1073858411385469. Neuroscientist. 2011. PMID: 22130639 Free PMC article. Review. - Conformational templating of α-synuclein aggregates in neuronal-glial cultures.
Sacino AN, Thomas MA, Ceballos-Diaz C, Cruz PE, Rosario AM, Lewis J, Giasson BI, Golde TE. Sacino AN, et al. Mol Neurodegener. 2013 May 28;8:17. doi: 10.1186/1750-1326-8-17. Mol Neurodegener. 2013. PMID: 23714769 Free PMC article.
References
- Simuni T, Hurtig HI. Parkinson's Disease: the Clinical Picture. In: Clark CM, Trojanoswki JQ, editors. Neurodegenerative dementias. New York: McGraw-Hill; 2000. pp. 193–203.
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999;56:33–39. - PubMed
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(Pt 8):1437–1448. - PubMed
- Holdorff B. Fritz Heinrich Lewy (1885–1950) J. Neurol. 253;2006:677–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG009215/AG/NIA NIH HHS/United States
- NS053488/NS/NINDS NIH HHS/United States
- P50 NS053488-020004/NS/NINDS NIH HHS/United States
- T32 AG000255/AG/NIA NIH HHS/United States
- AG09215/AG/NIA NIH HHS/United States
- P50 NS053488/NS/NINDS NIH HHS/United States
- P50 NS053488-01A20004/NS/NINDS NIH HHS/United States
- P01 AG009215-200009/AG/NIA NIH HHS/United States
- T32 AG00255/AG/NIA NIH HHS/United States
- T32 AG000255-10/AG/NIA NIH HHS/United States
- P01 AG009215-18/AG/NIA NIH HHS/United States
- P01 AG009215-190009/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical