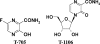Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106 - PubMed (original) (raw)
Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106
Justin G Julander et al. Antimicrob Agents Chemother. 2009 Jan.
Abstract
Treatment with the nucleoside analog T-1106 was previously shown to be effective in a hamster model of yellow fever virus (YFV) disease, even though it had only slight activity in cell culture. In the study described in this report, the activity of T-705, a chemically related compound currently undergoing clinical trials for the treatment of influenza (FDANews 4:1, 2007), was tested against YFV in cell culture and in the hamster model. The antiviral efficacy of T-705 in cell culture occurred at a concentration of 330 microM, which was more than threefold lower than the concentration at which T-1106 had antiviral efficacy, as determined by a virus yield reduction assay and confirmed by a luciferase-based ATP detection assay. Time-of-addition studies revealed that addition of T-705, T-1106, or ribavirin at 0, 4, 8, or 12 h after virus challenge was effective in inhibiting virus in Vero cells, suggesting that these three agents have similar mechanisms of action in cell culture. Because of its more potent activity in cell culture, it was anticipated that T-705 treatment of hamsters infected with YFV would result in protection from disease. Significant improvements in survival and disease parameters were seen in infected animals when T-705 was administered orally at a dose of 200 or 400 mg/kg of body weight per day when it was given twice a day for 8 days. Significant improvements were also observed with a dose of 400 mg/kg/day when treatment initiation was delayed as late as 3 days after virus inoculation. Although the dose of T-705 required for efficacy in hamsters is higher than that of T-1106 required for efficacy, T-705 treatment is effective in significantly improving disease parameters in YFV-infected hamsters, which may indicate its potential utility in the treatment of YFV disease in humans.
Figures
FIG. 1.
Structures of T-705 and T-1106.
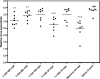
FIG. 2.
Time-of-addition study, in which T-705, T-1106, or ribavirin was added 0, 4, 8, 12, or 16 h after virus attachment. The supernatant was assayed for the initial level of virus production at 24 h after virus attachment by a virus yield reduction assay, and the results were compared with those of experiments in which no compound was added. Various doses of compound were tested at each treatment initiation time point, and the resulting CCID50s are shown. Tests were run in duplicate, and mean values are shown. Two independent experiments were conducted, and representative data are shown.
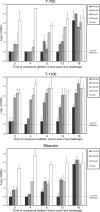
FIG. 3.
YFV RNA levels detected by QRT-PCR on 6 dpi from liver tissue of hamsters treated with various concentrations of T-705, T-1106, or ribavirin. mpk, mg/kg/day; ***, P < 0.001 compared with the results for the placebo group; **, P < 0.01 compared with the results for the placebo group.
Similar articles
- Activity of T-1106 in a hamster model of yellow Fever virus infection.
Julander JG, Furuta Y, Shafer K, Sidwell RW. Julander JG, et al. Antimicrob Agents Chemother. 2007 Jun;51(6):1962-6. doi: 10.1128/AAC.01494-06. Epub 2007 Apr 9. Antimicrob Agents Chemother. 2007. PMID: 17420215 Free PMC article. - Efficacy of 2'-C-methylcytidine against yellow fever virus in cell culture and in a hamster model.
Julander JG, Jha AK, Choi JA, Jung KH, Smee DF, Morrey JD, Chu CK. Julander JG, et al. Antiviral Res. 2010 Jun;86(3):261-7. doi: 10.1016/j.antiviral.2010.03.004. Epub 2010 Mar 20. Antiviral Res. 2010. PMID: 20227442 Free PMC article. - AT-752, a double prodrug of a guanosine nucleotide analog, inhibits yellow fever virus in a hamster model.
Lin K, Good SS, Julander JG, Weight AE, Moussa A, Sommadossi JP. Lin K, et al. PLoS Negl Trop Dis. 2022 Jan 24;16(1):e0009937. doi: 10.1371/journal.pntd.0009937. eCollection 2022 Jan. PLoS Negl Trop Dis. 2022. PMID: 35073319 Free PMC article. - T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections.
Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, Gowen BB, Julander JG, Morrey JD. Furuta Y, et al. Antiviral Res. 2009 Jun;82(3):95-102. doi: 10.1016/j.antiviral.2009.02.198. Epub 2009 Mar 6. Antiviral Res. 2009. PMID: 19428599 Free PMC article. Review. - Treatment of yellow fever.
Monath TP. Monath TP. Antiviral Res. 2008 Apr;78(1):116-24. doi: 10.1016/j.antiviral.2007.10.009. Epub 2007 Nov 20. Antiviral Res. 2008. PMID: 18061688 Review.
Cited by
- Zika Virus Replication Is Substantially Inhibited by Novel Favipiravir and Interferon Alpha Combination Regimens.
Pires de Mello CP, Tao X, Kim TH, Bulitta JB, Rodriquez JL, Pomeroy JJ, Brown AN. Pires de Mello CP, et al. Antimicrob Agents Chemother. 2017 Dec 21;62(1):e01983-17. doi: 10.1128/AAC.01983-17. Print 2018 Jan. Antimicrob Agents Chemother. 2017. PMID: 29109164 Free PMC article. - Targeting viral genome synthesis as broad-spectrum approach against RNA virus infections.
Huchting J. Huchting J. Antivir Chem Chemother. 2020 Jan-Dec;28:2040206620976786. doi: 10.1177/2040206620976786. Antivir Chem Chemother. 2020. PMID: 33297724 Free PMC article. Review. - Nucleoside analogs as a rich source of antiviral agents active against arthropod-borne flaviviruses.
Eyer L, Nencka R, de Clercq E, Seley-Radtke K, Růžek D. Eyer L, et al. Antivir Chem Chemother. 2018 Jan-Dec;26:2040206618761299. doi: 10.1177/2040206618761299. Antivir Chem Chemother. 2018. PMID: 29534608 Free PMC article. Review. - Antiviral activity of ribavirin and favipiravir against human astroviruses.
Janowski AB, Dudley H, Wang D. Janowski AB, et al. J Clin Virol. 2020 Feb;123:104247. doi: 10.1016/j.jcv.2019.104247. Epub 2019 Dec 17. J Clin Virol. 2020. PMID: 31864069 Free PMC article. - Rifapentine is an entry and replication inhibitor against yellow fever virus both in vitro and in vivo.
Qian X, Wu B, Tang H, Luo Z, Xu Z, Ouyang S, Li X, Xie J, Yi Z, Leng Q, Liu Y, Qi Z, Zhao P. Qian X, et al. Emerg Microbes Infect. 2022 Dec;11(1):873-884. doi: 10.1080/22221751.2022.2049983. Emerg Microbes Infect. 2022. PMID: 35249454 Free PMC article.
References
- Bae, H. G., C. Drosten, P. Emmerich, R. Colebunders, P. Hantson, S. Pest, M. Parent, H. Schmitz, M. A. Warnat, and M. Niedrig. 2005. Analysis of two imported cases of yellow fever infection from Ivory Coast and The Gambia to Germany and Belgium. J. Clin. Virol. 33:274-280. - PubMed
- Bearcroft, W. G. 1960. Electron-microscope studies on the liver cells of yellow-fever-infected rhesus monkeys. J. Pathol. Bacteriol. 80:421-426. - PubMed
- Centers for Disease Control and Prevention. 2002. Adverse events associated with 17D-derived yellow fever vaccination—United States, 2001-2002. MMWR Morb. Mortal. Wkly. Rep. 51:989-993. - PubMed
- FDANews. 2007. Toyama starts U.S. trials of polymerase inhibitor. RxTrials Institute drug pipeline alert. FDANews 4:1.
Publication types
MeSH terms
Substances
Grants and funding
- N01 AI015435/AI/NIAID NIH HHS/United States
- N01 AI030048/AI/NIAID NIH HHS/United States
- N01-AI-15435/AI/NIAID NIH HHS/United States
- N01-AI-30048/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources