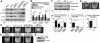Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway - PubMed (original) (raw)
Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway
Panagiotis Flevaris et al. Blood. 2009.
Abstract
Mitogen-activated protein kinases (MAPK), p38, and extracellular stimuli-responsive kinase (ERK), are acutely but transiently activated in platelets by platelet agonists, and the agonist-induced platelet MAPK activation is inhibited by ligand binding to the integrin alpha(IIb)beta(3). Here we show that, although the activation of MAPK, as indicated by MAPK phosphorylation, is initially inhibited after ligand binding to integrin alpha(IIb)beta(3), integrin outside-insignaling results in a late but sustained activation of MAPKs in platelets. Furthermore, we show that the early agonist-induced MAPK activation and the late integrin-mediated MAPK activation play distinct roles in different stages of platelet activation. Agonist-induced MAPK activation primarily plays an important role in stimulating secretion of platelet granules, while integrin-mediated MAPK activation is important in facilitating clot retraction. The stimulatory role of MAPK in clot retraction is mediated by stimulating myosin light chain (MLC) phosphorylation. Importantly, integrin-dependent MAPK activation, MAPK-dependent MLC phosphorylation, and clot retraction are inhibited by a Rac1 inhibitor and in Rac1 knockout platelets, indicating that integrin-induced activation of MAPK and MLC and subsequent clot retraction is Rac1-dependent. Thus, our results reveal 2 different activation mechanisms of MAPKs that are involved in distinct aspects of platelet function and a novel Rac1-MAPK-dependent cell retractile signaling pathway.
Figures
Figure 1
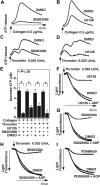
The effect of p38 and MEK inhibitors on platelet secretion and aggregation. (A,B) Washed platelets were preincubated at 37°C for 2 minutes in the presence of either 10 μM p38 inhibitor, SB203580 (A), 3 μM MEK inhibitor, U0126 (B), or DMSO vehicle control. Platelet aggregation was induced by 0.5 μg/mL collagen in the presence of luciferase reagent, and ATP release was measured using a platelet lumi-aggregometer. (C,D) Washed platelets were preincubated with either SB203580 (C), U0126 (D), or DMSO. Platelets were stimulated with 0.025 U/mL thrombin, and ATP release was measured. (E) Quantification of peak platelet ATP release (in μM, mean ± SD). Statistical significance was determined using Student t test. Platelets were preincubated with either 10 μM SB203580, 3 μM U0126, or vehicle control (F,G); or 30 μM SB203580, PD98059, or vehicle control (H,I), and stimulated with 0.025 U/mL thrombin in the presence or absence of 0.5 μM ADP in a turbdometric aggregometer. Aggregation traces are shown.
Figure 2
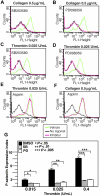
The effect of p38, MEK inhibitors, and aspirin on thrombin and collagen-induced p-selectin expression. Washed human platelets were resuspended in Tyrode buffer and preincubated with either 20 μM SB203580 (A,C), 10 μM MEK inhibitor, PD98059 (B,D), 1 mM aspirin (E,F), or the appropriate vehicle control for 5 minutes. Platelets were then treated with either 0.5 μg/mL collagen (A,B,F) or 0.025 U/mL thrombin (C-E) at 37°C for 5 minutes and fixed by adding paraformaldehyde. Fixed platelets were incubated with the monoclonal anti–human p-selectin antibody SZ51 at 22°C for 30 minutes and, after washing, were further incubated with a FITC-conjugated goat anti–mouse Ig antibody. Surface expression of the α-granule membrane protein p-selectin was analyzed using flow cytometry. (G) Quantitative results from 3 experiments are expressed as the p-selectin expression index (fluorescence intensity of platelets stimulated with an agonist/fluorescence intensity of unstimulated platelets). Statistical differences between inhibitor-treated samples and controls were examined by Student t test.
Figure 3
Kinetics of activation of p38, ERK, and MLC in platelets spreading on fibrinogen. Washed platelets at a concentration of 4 × 107/mL were incubated with 500 μM aspirin and 50 μM P2Y12 inhibitor, 2MeSAMP for 5 minutes at 37°C. Platelets were added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for indicated times or kept in suspension. Dishes were rinsed to remove nonadherent platelets, and platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation in platelets.” Activation of p38, ERK, and MLC were analyzed by Western blot analysis using phospho-specific antibodies against phosphorylated Thr180/Tyr182 (p38), Thr-202/Tyr204 (ERK), and Thr18/Ser19 (MLC) (Cell Signaling Technologies). Monoclonal antibody against tubulin was used to verify equal loading.
Figure 4
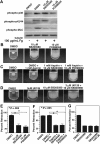
p38 and MEK inhibitors inhibit MLC phosphorylation and clot retraction. (A) Washed platelets at a concentration of 4 × 107/mL were preincubated with 500 μM aspirin and with 50 μM P2Y12 inhibitor, 2MeSAMP for 5 minutes at 37°C. Platelets were also pretreated with either 10 μM SB203580, 10 μM PD98059, or equal volume DMSO for 30 minutes at 25°C and added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for 60 minutes, or kept in suspension. After washing to remove nonadherent platelets, adherent platelets or control platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation in platelets.” Phosphorylation of p38, ERK, and MLC were analyzed by Western blot analysis using specific antibodies against phosphorylated p38, ERK, and MLC. A monoclonal antibody against tubulin was used to verify equal loading. (B,C) Platelet-rich plasma was anticoagulated with 0.38% sodium citrate and preincubated with (C) or without (B) 1 mM aspirin for 5 minutes at 25°C and with p38 and MEK inhibitor as indicated. Coagulation was induced with 0.2 U/mL thrombin, and clots were allowed to retract at 37°C and photographed. (D) Platelet-rich plasma was preincubated with either vehicle control, 10 μM SB203580, 3 μM U0126, or both inhibitors. Coagulation was induced with 0.2 U/mL thrombin, and clots were allowed to retract at 37°C and photographed. (E-G) Two-dimensional retraction of clots in panels B through D were measured using NIH ImageJ and expressed as percent retraction (mean ± SD). Statistical significance was determined using a Student t test.
Figure 5
The effects of Rac1 inhibition and ROCK inhibition on p38, ERK, and MLC phosphorylation and clot retraction. (A)Washed platelets were pretreated with either 20 μM NSC23766, 20 μM ROCK inhibitor, Y27632, both NSC23766 and Y27632, or the appropriate vehicle control for 30 minutes at 25°C. Platelets were added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for 60 minutes or kept in suspension. After washing, adherent platelets or control platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation platelets.” Phosphorylation of p38, ERK, and MLC were analyzed by Western blot analysis using specific antibodies against phosphorylated p38, ERK, and MLC. A monoclonal antibody against tubulin was used to verify equal loading. (B) Densitometry measurements from results in panel A. Values were normalized with respect to resting control for each immunoblot and are expressed as relative phosphorylation (mean ± SD from 3 separate experiments). Statistical significance was determined using Student t test. (C,D) Citrated PRP was preincubated with inhibitors for 30 minutes as indicated. Coagulation was induced with 0.2 U/mL thrombin, and clots were allowed to retract at 37°C and photographed. (E) Washed platelets from wild-type and Rac1 knockout mice were solubilized, and expression of Rac1 was analyzed by immunoblot with monoclonal antibody against Rac1 (Sigma-Aldrich). (F) Washed platelets from wild-type, and Rac1 knockout mice were added to polystyrene dishes coated with 100 μg/mL fibrinogen to induce adhesion and spreading at 37°C for 60 minutes or kept in suspension. After washing, adherent platelets or control platelets were solubilized as described in “Immunoblot detection of p38, ERK, and MLC phosphorylation in platelets.” Phosphorylation of p38 and ERK were analyzed by Western blot analysis. A monoclonal antibody against tubulin was used to verify equal loading. (G) Platelets from wild-type or Rac1 knockout mice were mixed with human platelet-poor plasma at a concentration of 4 × 108/mL, induced to coagulate with 0.4 U/mL thrombin, and photographed. (H-J) Extent of clot retraction in panels C, D, and G was measured using NIH ImageJ and expressed as percent retraction (mean ± SD). Statistical significance was determined using a Student t test.
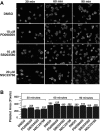
Figure 6
p38, MEK, and Rac1 inhibitors do not affect platelet spreading on fibrinogen. (A) Platelets were preincubated with either 10 μM SB203580, 10 μM PD98059, 20 μM rac 1 inhibitor, NSC23766, or DMSO for 30 minutes at 25°C. Platelets were allowed to spread on fibrinogen-coated slides for 30, 60, and 90 minutes, and were fixed with paraformaldehyde to stop spreading. Platelets were subsequently labeled with FITC-conjugated phalloidin and photographed using a fluorescence microscope. (B) Quantification of area (pixel number) in 4 random fields (mean ± SE; platelet numbers are marked above each column). Statistical analysis performed using Student t test. At all 3 time points, no statistical significance between groups was found.
Similar articles
- Ginsenoside-Rp3 inhibits platelet activation and thrombus formation by regulating MAPK and cyclic nucleotide signaling.
Irfan M, Jeong D, Kwon HW, Shin JH, Park SJ, Kwak D, Kim TH, Lee DH, Park HJ, Rhee MH. Irfan M, et al. Vascul Pharmacol. 2018 Oct;109:45-55. doi: 10.1016/j.vph.2018.06.002. Epub 2018 Jun 8. Vascul Pharmacol. 2018. PMID: 29890296 - Distinct roles for Rap1b protein in platelet secretion and integrin αIIbβ3 outside-in signaling.
Zhang G, Xiang B, Ye S, Chrzanowska-Wodnicka M, Morris AJ, Gartner TK, Whiteheart SW, White GC 2nd, Smyth SS, Li Z. Zhang G, et al. J Biol Chem. 2011 Nov 11;286(45):39466-77. doi: 10.1074/jbc.M111.239608. Epub 2011 Sep 22. J Biol Chem. 2011. PMID: 21940635 Free PMC article. - Biphasic myosin II light chain activation during clot retraction.
Egot M, Kauskot A, Lasne D, Gaussem P, Bachelot-Loza C. Egot M, et al. Thromb Haemost. 2013 Dec;110(6):1215-22. doi: 10.1160/TH13-04-0335. Epub 2013 Aug 22. Thromb Haemost. 2013. PMID: 23965920 - Fine-Tuning of Platelet Responses by Serine/Threonine Protein Kinases and Phosphatases-Just the Beginning.
Shiravand Y, Walter U, Jurk K. Shiravand Y, et al. Hamostaseologie. 2021 Jun;41(3):206-216. doi: 10.1055/a-1476-7873. Epub 2021 Jun 30. Hamostaseologie. 2021. PMID: 34192779 Review. - Platelet MAPKs-a 20+ year history: What do we really know?
Patel P, Naik UP. Patel P, et al. J Thromb Haemost. 2020 Sep;18(9):2087-2102. doi: 10.1111/jth.14967. Epub 2020 Jul 23. J Thromb Haemost. 2020. PMID: 32574399 Review.
Cited by
- Platelet gene expression and function in patients with COVID-19.
Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, Petrey AC, Tolley ND, Guo L, Cody M, Weyrich AS, Yost CC, Rondina MT, Campbell RA. Manne BK, et al. Blood. 2020 Sep 10;136(11):1317-1329. doi: 10.1182/blood.2020007214. Blood. 2020. PMID: 32573711 Free PMC article. - Targeting integrin pathways: mechanisms and advances in therapy.
Pang X, He X, Qiu Z, Zhang H, Xie R, Liu Z, Gu Y, Zhao N, Xiang Q, Cui Y. Pang X, et al. Signal Transduct Target Ther. 2023 Jan 2;8(1):1. doi: 10.1038/s41392-022-01259-6. Signal Transduct Target Ther. 2023. PMID: 36588107 Free PMC article. Review. - Streptococcus sanguinis-induced cytokine and matrix metalloproteinase-1 release from platelets.
Cognasse F, Hamzeh-Cognasse H, Chabert A, Jackson E, Arthaud CA, Garraud O, McNicol A. Cognasse F, et al. BMC Immunol. 2014 Apr 22;15:15. doi: 10.1186/1471-2172-15-15. BMC Immunol. 2014. PMID: 24755160 Free PMC article. - Secretory phospholipase A2 modified HDL rapidly and potently suppresses platelet activation.
Curcic S, Holzer M, Pasterk L, Knuplez E, Eichmann TO, Frank S, Zimmermann R, Schicho R, Heinemann A, Marsche G. Curcic S, et al. Sci Rep. 2017 Aug 14;7(1):8030. doi: 10.1038/s41598-017-08136-1. Sci Rep. 2017. PMID: 28808297 Free PMC article. - New Therapeutic Agent against Arterial Thrombosis: An Iridium(III)-Derived Organometallic Compound.
Hsia CW, Velusamy M, Tsao JT, Hsia CH, Chou DS, Jayakumar T, Lee LW, Li JY, Sheu JR. Hsia CW, et al. Int J Mol Sci. 2017 Dec 5;18(12):2616. doi: 10.3390/ijms18122616. Int J Mol Sci. 2017. PMID: 29206177 Free PMC article.
References
- Saklatvala J, Rawlinson L, Waller RJ, et al. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J Biol Chem. 1996;271:6586–6589. - PubMed
- Kramer RM, Roberts EF, Strifler BA, Johnstone EM. Thrombin induces activation of p38 MAP kinase in human platelets. J Biol Chem. 1995;270:27395–27398. - PubMed
- Nadal F, Levy-Toledano S, Grelac F, Caen JP, Rosa JP, Bryckaert M. Negative regulation of mitogen-activated protein kinase activation by integrin αIIbβ3 in platelets. J Biol Chem. 1997;272:22381–22384. - PubMed
- Bugaud F, Nadal-Wollbold F, Levy-Toledano S, Rosa JP, Bryckaert M. Regulation of c-jun-NH2 terminal kinase and extracellular-signal regulated kinase in human platelets. Blood. 1999;94:3800–3805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL062350/HL/NHLBI NIH HHS/United States
- R01 HL080264/HL/NHLBI NIH HHS/United States
- R01 HL062350/HL/NHLBI NIH HHS/United States
- HL068819/HL/NHLBI NIH HHS/United States
- R01 HL068819/HL/NHLBI NIH HHS/United States
- HL080264/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous