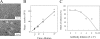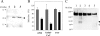Protease-mediated entry via the endosome of human coronavirus 229E - PubMed (original) (raw)
Protease-mediated entry via the endosome of human coronavirus 229E
Miyuki Kawase et al. J Virol. 2009 Jan.
Abstract
Human coronavirus 229E, classified as a group I coronavirus, utilizes human aminopeptidase N (APN) as a receptor; however, its entry mechanism has not yet been fully elucidated. We found that HeLa cells infected with 229E via APN formed syncytia when treated with trypsin or other proteases but not in a low-pH environment, a finding consistent with syncytium formation by severe acute respiratory syndrome coronavirus (SARS-CoV). In addition, trypsin induced cleavage of the 229E S protein. By using infectious viruses and pseudotyped viruses bearing the 229E S protein, we found that its infection was profoundly blocked by lysosomotropic agents as well as by protease inhibitors that also prevented infection with SARS-CoV but not that caused by murine coronavirus mouse hepatitis virus strain JHMV, which enters cells directly from the cell surface. We found that cathepsin L (CPL) inhibitors blocked 229E infection the most remarkably among a variety of protease inhibitors tested. Furthermore, 229E infection was inhibited in CPL knockdown cells by small interfering RNA, compared with what was seen for a normal counterpart producing CPL. However, its inhibition was not so remarkable as that found with SARS-CoV infection, which seems to indicate that while CPL is involved in the fusogenic activation of 229E S protein in endosomal infection, not-yet-identified proteases could also play a part in that activity. We also found 229E virion S protein to be cleaved by CPL. Furthermore, as with SARS-CoV, 229E entered cells directly from the cell surface when cell-attached viruses were treated with trypsin. These findings suggest that 229E takes an endosomal pathway for cell entry and that proteases like CPL are involved in this mode of entry.
Figures
FIG. 1.
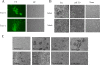
Syncytium formation of 229E-infected HeLa cells induced by trypsin and other protease treatment. (A) HeLa cells infected with 229E were cultured for 2 days and then treated or left untreated with trypsin (Try; 100 μg/ml) at RT for 5 min. Cells were cultured for an additional 2 h and observed for syncytium formation and viral antigen via phase-contrast (PC) and fluorescent (FA) microscopy, respectively. (B) Cells infected (Infect) with 229E or mock infected (Mock) were cultured for 2 days and treated with 100 μg/ml of trypsin (Try) or DMEM adjusted to pH 5.0 at RT for 5 min. After 2 h of culture, cells were fixed with formaldehyde and stained with crystal violet. Cells without any treatment were shown as controls (None). (C) Syncytium formation of cells infected with 229E was induced by treatment with various proteases. HeLa cells infected with 229E and cultured for 2 days were treated with a variety of proteases at RT for 5 min and cultured for an additional 2 h. Cells were fixed and stained as described above. The following concentrations were employed for treatment: for proteinase K, 8 μg/ml; for elastase, 2 mg/ml; for thermolysin, 200 μg/ml; for dispase, 1 unit/ml; for papain, 0.19 unit/ml; for chymotrypsin, 2 mg/ml; and for collagenase, 1 mg/ml. Protease (−) indicates the absence of protease.
FIG. 2.
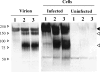
Western blotting analysis of the 229E S protein after treatment with trypsin. (Left) 229E virions concentrated as described in Materials and Methods were treated with trypsin (lane 1, 0 μg/ml; lane 2, 50 μg/ml; lane 3, 100 μg/ml) at RT for 5 min. (Right) Also, 229E-infected and mock-infected HeLa cells were treated with trypsin (lanes 1, 0 μg/ml; lanes 2, 250 μg/ml; lanes 3, 500 μg/ml). The resultant samples were analyzed by Western blotting using anti-229E S antibodies raised with a synthetic peptide corresponding to the C-terminal region of the S protein. Uncleaved S protein and cleaved S2 protein are indicated by black and white arrowheads, respectively.
FIG. 3.
Characterization of HeLa cells permissive for 229E infection. (A) HeLa cells infected with 229E and cultured for 1 day in the presence or absence of trypsin (Try; 5 μg/ml) in DMEM containing 10% TPB were fixed and stained as described above. The syncytia formed, indicated by arrows, were observed under microscopy as plaques. (B) To evaluate the plaque assay developed using HeLa cells and trypsin, 229E virion solutions at ca. 120 PFU/50 μl were diluted by twofold steps and inoculated into 3 wells of HeLa cells prepared in 24-well plates. The relationship between the virus dilution and the plaque number is depicted. (C) HeLa cells prepared in 96-well plates were treated with anti-human APN sera at 4°C for 45 min and then allowed to be adsorbed with ca. 150 PFU of 229E at 4°C for 45 min in the presence of antiserum. Next, those cells were cultured for 1 day in the presence of the antiserum. The infected cells were counted under fluorescence microscopy after cells had been stained with antibody (Ab) against 229E and secondary fluorescein isothiocyanate-labeled anti-rabbit serum. Cells in which infection was blocked by APN antibody were compared to those without APN antibody treatment.
FIG. 4.
Effect of lysosomotropic agents on 229E infection. (A) HeLa cells in 24-well plates were treated with 100 nM of Baf from 1 h before infection to 4 h postinfection (early) or from 4 to 24 h after 229E infection (late). Virus titers were determined for cells at 24 h postinfection. HeLa cells were infected at an MOI of 1. (B) HeLa-AC cells prepared in 24-well plates were treated with 1,000 nM of Baf or 50 mM NH4Cl for 1 h at 37°C. Then, cells were infected with 1 × 105 PFU of virus (229E, MHV-JHM, or MHV-2) in DMEM containing each agent and incubated at 34°C (229E) or 37°C (MHV) for 1 h. After being washed once, cells were cultured with DMEM containing each agent for 3 h, and then medium was changed to DMEM without agent. Virus titers were examined by plaque assay in cells at 24 h (229E) or 10 h (MHV) after infection. (C) HeLa-A cells prepared in 96-well plates were treated with different concentrations of Baf or NH4Cl for 1 h; infected with ca. 500 IU of pseudotyped VSV bearing 229E S (solid line with circle), SARS-CoV S (broken line with square), or VSV G protein (dotted line with triangle); and cultured for 24 h in the presence of each agent. Then, GFP-positive cells were photographed by Keyence fluorescence microscopy and counted. The percentage of infection was calculated as follows: (GFP-positive cell number in the presence of agent)/(GFP-positive cell number in the absence of agent) × 100. Cr, control.
FIG. 5.
Effects of protease inhibitors on pseudotyped virus infection. HeLa-A cells pretreated with different concentrations of protease inhibitors for 1 h were infected with ca. 500 IU of pseudotyped VSV bearing 229E S (solid line with circle); SARS-CoV S (broken line with square) or VSV G protein (dotted line with triangle) and cultured at 37°C for 24 h in the presence of protease inhibitors. The percentage of infection was determined as described for Fig. 4C. MDL, MDL28107.
FIG. 6.
Effect of CPL knockdown by siRNA on pseudotype infection (A and B) and digestion of 229E S by CPL (C). (A) HeLa-A cells were treated with siRNA for CPL (lane 1) and control nonsense RNA (lane 2) or were left untreated (lane 3) and cultured for 4 days. Amounts of CPL expressed in those cells were examined by Western blotting. The arrowhead indicates the position of CPL. (B) HeLa-A cells transfected with CPL siRNA and cultured for 4 days (black columns) were infected with ca. 500 IU of pseudotyped VSV with 229E S, SARS-CoV S, or VSV G protein. Their infection rates (GFP-positive cell numbers) were compared to those for cells transfected with control RNA (gray columns). (C) 229E virions concentrated as described in Materials and Methods were treated with 36 μg/ml of CPL (lane 2) or CPB (lane 3) or were mock treated (lane 1) at 37°C for 30 min in a buffer at pH 5.0, and S protein was analyzed by Western blotting using anti-229E S antibodies as described in the Fig. 2 legend. As a control, virions were also treated (lane 5) or mock treated (lane 4) with 50 μg/ml of trypsin at 37°C for 30 min in a buffer at pH. 70 and analyzed by Western blotting. M, molecular mass.
FIG. 7.
Infection with 229E S-bearing pseudotyped VSV from cell surfaces. HeLa-A cells prepared in 96-well plates were treated with 100 nM Baf for 1 h at 37°C and then allowed to adsorb VSV bearing 229E S, SARS-CoV S, or VSV G protein on ice for 30 min. The plate was left at RT for 10 min, and adsorbed virus was treated with 100 μg/ml trypsin for 5 min. Then, those cells were cultured for 24 h at 37°C, and GFP-positive cell numbers were calculated as described above (Baf positive, Try positive). GFP-positive cell numbers were also determined for the groups of cells treated with Baf alone (Baf +, Try −) or trypsin alone (Baf −, Try +) or maintained as untreated control cells (Baf −, Try −). The number of GFP-positive cells in each group is shown as a percentage in comparison with that of GFP-positive cells without any treatment.
Similar articles
- Clinical Isolates of Human Coronavirus 229E Bypass the Endosome for Cell Entry.
Shirato K, Kanou K, Kawase M, Matsuyama S. Shirato K, et al. J Virol. 2016 Dec 16;91(1):e01387-16. doi: 10.1128/JVI.01387-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27733646 Free PMC article. - Characterization of spike S1/S2 processing and entry pathways of lentiviral pseudoviruses bearing seasonal human coronaviruses NL63, 229E, and HKU1 spikes.
Neerukonda SN, Vassell R, Lusvarghi S, Liu S, Akue A, Kukuruga M, Wang TT, Weiss CD, Wang W. Neerukonda SN, et al. Microbiol Spectr. 2025 Mar 4;13(3):e0280824. doi: 10.1128/spectrum.02808-24. Epub 2025 Jan 28. Microbiol Spectr. 2025. PMID: 39873512 Free PMC article. - Entry from the cell surface of severe acute respiratory syndrome coronavirus with cleaved S protein as revealed by pseudotype virus bearing cleaved S protein.
Watanabe R, Matsuyama S, Shirato K, Maejima M, Fukushi S, Morikawa S, Taguchi F. Watanabe R, et al. J Virol. 2008 Dec;82(23):11985-91. doi: 10.1128/JVI.01412-08. Epub 2008 Sep 10. J Virol. 2008. PMID: 18786990 Free PMC article. - [Protease-dependent cell entry mechanism of coronaviruses].
Matsuyama S. Matsuyama S. Uirusu. 2011 Jun;61(1):109-16. doi: 10.2222/jsv.61.109. Uirusu. 2011. PMID: 21972562 Review. Japanese. - [Cell entry mechanisms of coronaviruses].
Taguchi F, Matsuyama S. Taguchi F, et al. Uirusu. 2009 Dec;59(2):215-22. doi: 10.2222/jsv.59.215. Uirusu. 2009. PMID: 20218330 Review. Japanese.
Cited by
- Receptor-bound porcine epidemic diarrhea virus spike protein cleaved by trypsin induces membrane fusion.
Park JE, Cruz DJ, Shin HJ. Park JE, et al. Arch Virol. 2011 Oct;156(10):1749-56. doi: 10.1007/s00705-011-1044-6. Epub 2011 Jun 12. Arch Virol. 2011. PMID: 21667287 Free PMC article. - Clathrin- and serine proteases-dependent uptake of porcine epidemic diarrhea virus into Vero cells.
Park JE, Cruz DJ, Shin HJ. Park JE, et al. Virus Res. 2014 Oct 13;191:21-9. doi: 10.1016/j.virusres.2014.07.022. Epub 2014 Jul 31. Virus Res. 2014. PMID: 25086180 Free PMC article. - The role of cysteine peptidases in coronavirus cell entry and replication: The therapeutic potential of cathepsin inhibitors.
Pišlar A, Mitrović A, Sabotič J, Pečar Fonović U, Perišić Nanut M, Jakoš T, Senjor E, Kos J. Pišlar A, et al. PLoS Pathog. 2020 Nov 2;16(11):e1009013. doi: 10.1371/journal.ppat.1009013. eCollection 2020 Nov. PLoS Pathog. 2020. PMID: 33137165 Free PMC article. Review. - Jamaican fruit bat (Artibeus jamaicensis) insusceptibility to mucosal inoculation with SARS-CoV-2 Delta variant is not caused by receptor compatibility.
Port JR, Riopelle JC, van Tol S, Wickenhagen A, Bohrnsen E, Sturdevant DE, Rosenke R, Lovaglio J, Lack J, Anzick SL, Cordova K, Yinda KC, Hanley PW, Schountz T, Kendall LV, Shaia CI, Saturday G, Martens C, Schwarz B, Munster VJ. Port JR, et al. Npj Viruses. 2024 Jul 16;2(1):26. doi: 10.1038/s44298-024-00037-1. Npj Viruses. 2024. PMID: 40295878 Free PMC article. - Neutralization interfering antibodies: a "novel" example of humoral immune dysfunction facilitating viral escape?
Nicasio M, Sautto G, Clementi N, Diotti RA, Criscuolo E, Castelli M, Solforosi L, Clementi M, Burioni R. Nicasio M, et al. Viruses. 2012 Sep;4(9):1731-52. doi: 10.3390/v4091731. Epub 2012 Sep 24. Viruses. 2012. PMID: 23170181 Free PMC article. Review.
References
- Ami, Y., N. Nagata, K. Shirato, R. Watanabe, N. Iwata, K. Nakagaki, S. Fukushi, M. Saijo, S. Morikawa, and F. Taguchi. 2008. Co-infection of respiratory bacterium with severe acute respiratory syndrome coronavirus induces an exacerbated pneumonia in mice. Microbiol. Immunol. 52118-127. - PMC - PubMed
- Appleyard, G., and M. Tisdale. 1985. Inhibition of the growth of human coronavirus 229E by leupeptin. J. Gen. Virol. 66363-366. - PubMed
- Bosch, B. J., B. E. Martina, R. Van Der Zee, J. Lepault, B. J. Haijema, C. Versluis, A. J. Heck, R. De Groot, A. D. Osterhaus, and P. J. Rottier. 2004. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. USA 1018455-8460. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous