COPI coat assembly occurs on liquid-disordered domains and the associated membrane deformations are limited by membrane tension - PubMed (original) (raw)
COPI coat assembly occurs on liquid-disordered domains and the associated membrane deformations are limited by membrane tension
Jean-Baptiste Manneville et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Cytoplasmic coat proteins are required for cargo selection and budding of tubulovesicular transport intermediates that shuttle between intracellular compartments. To better understand the physical parameters governing coat assembly and coat-induced membrane deformation, we have reconstituted the Arf1-dependent assembly of the COPI coat on giant unilamellar vesicles by using fluorescently labeled Arf1 and coatomer. Membrane recruitment of Arf1-GTP occurs exclusively on disordered lipid domains and does not induce optically visible membrane deformation. In the presence of Arf1-GTP, coatomer self-assembles into weakly curved coats on membranes under high tension, while it induces extensive membrane deformation at low membrane tension. These deformations appear to have a composition different from the parental membrane because they are protected from phase transition. These findings suggest that the COPI coat is adapted to liquid disordered membrane domains where it could promote lipid sorting and that its mechanical effects can be tuned by membrane tension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
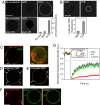
Sequential recruitment of Arf1 and coatomer on giant unilamellar vesicles (GUVs). (A) GUVs were incubated with 0.5 μM Arf1-OG in HKM buffer supplemented with GTP or GDP (0.1 mM). The ‘no nucleotide’ and ‘GDP’ images are shown at higher illumination intensity (‘laser 25×’, lower row). (Scale bar, 20 μm). (B) GUVs grown from Golgi mix supplemented with 1.5% (mol/mol) lipopeptide Lp23 were injected in a chamber containing 0.5 μM Arf1 and 0.15 μM coatomer-TMR in HKM buffer and either 0.1 mM GTP or 0.1 mM GDP. In A and B, quantification of membrane binding of Arf1-OG and of coatomer-TMR was determined from 30 to 35 vesicles in three independent experiments (mean ± SEM). (Scale bar, 20 μm). (C–F) Gallery of coatomer-coated GUVs. The experimental conditions were as in B. Depending on the GUV, coatomer (red) covers the whole surface (see B), forms static domains up to 5 μm in diameter (C) or concentrates in small (<1 μm in diameter) dynamic patches (D, arrows). Coatomer is also found associated with membrane deformations such as small dynamic budded profiles (arrows in E) or tubular profiles (arrows in F). The GUV membrane was labeled with 0.5% C5-HPC-BodipyFL (green). All images are confocal sections except the right panel in C, which is a 3D projection of a confocal stack. (Scale bars, 5 μm). (G) The mobility of coatomer assembled on tensed membranes is reduced compared with free Arf1-GTP and lipids. Averaged FRAP curves showing recovery of coatomer-TMR (red circles, n = 31), free Arf1-OG fluorescence (green squares, n = 31) and fluorescent lipids (black diamonds, n = 27). The photobleached region (see Inset) represents ≈20–30% of the total GUV area. The maximal recovery is thus ≈75% (dotted line). Error bars, SEM.
Fig. 2.
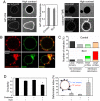
Low membrane tension facilitates coatomer-induced membrane deformation. (A) Streptolysin-O (SLO) was used to decrease membrane tension. Typical images of Arf1-OG (left side) and coatomer-TMR (right image) fluorescence on nontreated control GUVs and on GUVs in the presence of 10 μg/ml SLO are shown. Measurements pooled from 28–38 GUVs in three independent experiments (mean ± SEM) show that Arf1 binds to SLO-permeabilized GUVs to similar levels as in control experiments. High contrast images are provided to show the presence or absence of fluorescent proteins inside GUVs. Arf1-OG but not coatomer-TMR entered SLO-treated GUVs. (Scale bars, 10 μm). (B) Golgi mix GUVs with 1.5% (mol/mol) Lp23 were incubated in the presence of Arf1, coatomer-TMR, GTP as in Fig. 1_B_ and with 10 μg/ml SLO. The upper images show a GUV with numerous small (0.5–1 μm) and large (> 1 μm) deformation profiles. The lower images show a GUV with a zone of extensive membrane deformation. (Scale bar, 5 μm). (C) GUVs at high tension (control, upper graph) or at low tension (SLO, lower graph) were classified into four categories according to the size of the coatomer-coated regions and the extent of membrane deformations (see
SI Materials and Methods
). In the case of deformation profiles, the number of small (0.5–1 μm, _N_s) or large (> 1 μm, _N_l) profiles on the GUV was counted from a 3D confocal scan. GUVs were scored as displaying a low number of deformations if _N_s ≤ 5 or _N_l ≤ 5 (light green or light red, average numbers of deformations _N_s = 2.0 ± 0.5, _N_l = 1.6 ± 0.5 for control GUVs and _N_s = 3.6 ± 0.5, _N_l = 3.0 ± 0.5 for SLO-treated GUVs) or a high number of deformations if _N_s > 5 or _N_l >5 (dark green or dark red). Seventy-five vesicles from three independent experiments were scored in each condition. (D) Circularity (left image) and the deformation index (right image) were used to quantify membrane deformation (see
SI Materials and Methods
and inset in the right panel). Vesicles (13 to 63) from three independent experiments were scored in each condition (mean ± SEM.). Statistical difference between datasets is indicated (*, P < 0.001).
Fig. 3.
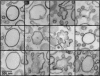
COPI-coated profiles on submicrometer liposomes. Golgi-mix liposomes with 2 mol% Lp23 were extruded through 0.4 μm polycarbonate filters. After incubation with Arf1, GTP, and coatomer, the sample was fixed with glutaraldehyde and the liposomes were collected by centrifugation and analyzed by thin section EM. The COPI-coated liposomes shown here were selected from the same liposome pellet (see
Fig. S9_B_
) and were ranked from left to right according to the extent of membrane deformation. (Scale bar, 200 nm)
Fig. 4.
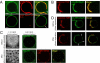
Arf1 and coatomer partition into liquid disordered (Ld) domains and protect Ld membranes from phase separation. (A) GUVs grown from sphingomyelin (BSM)/cholesterol/DOPC (3:1:3) mixtures exhibited phase separation, with μm-sized liquid ordered (Lo) domains surrounded with liquid-disordered (Ld) phase. Arf1-OG (Left) localized in the Ld phase (visualized with DHPE-TexasRed) and was excluded from Lo domains (lower image, visualized with cholera toxin-Cy3 and indicated by an arrow). (Scale bar, 5 μm). (B) On phase separated 3:2:1 GUVs and in the presence of Arf1-GTP and SLO, coatomer (red, left panel) specifically binds to and deforms Ld domains (visualized with C5-HPC-BodipyFL, green, middle panel). (C) Arf1 segregates in Ld domains and is excluded from Lo domains during phase separation. Phase separation was induced from initially homogeneous membranes (upper lane) by photobleaching 1:1:1 (left image) or 3:4:1 (Right) GUVs leading to the formation of Lo or Ld domains respectively (lower lane). (D) Phase separation occurs only in noncoated regions. Phase separation was induced by photobleaching a 1:1:1 GUV incubated with coatomer-TMR, Arf1, GTP, and SLO. Lo domains (arrows) were detected in the coatomer-free region of the GUV but not in the coatomer-coated area.
Similar articles
- Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation.
Antonny B, Bigay J, Casella JF, Drin G, Mesmin B, Gounon P. Antonny B, et al. Biochem Soc Trans. 2005 Aug;33(Pt 4):619-22. doi: 10.1042/BST0330619. Biochem Soc Trans. 2005. PMID: 16042557 Review. - Functional reconstitution of COPI coat assembly and disassembly using chemically defined components.
Reinhard C, Schweikert M, Wieland FT, Nickel W. Reinhard C, et al. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8253-7. doi: 10.1073/pnas.1432391100. Epub 2003 Jun 27. Proc Natl Acad Sci U S A. 2003. PMID: 12832619 Free PMC article. - Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport.
Presley JF, Ward TH, Pfeifer AC, Siggia ED, Phair RD, Lippincott-Schwartz J. Presley JF, et al. Nature. 2002 May 9;417(6885):187-93. doi: 10.1038/417187a. Nature. 2002. PMID: 12000962 - Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature.
Bigay J, Gounon P, Robineau S, Antonny B. Bigay J, et al. Nature. 2003 Dec 4;426(6966):563-6. doi: 10.1038/nature02108. Nature. 2003. PMID: 14654841 - Physical aspects of COPI vesicle formation.
Pinot M, Goud B, Manneville JB. Pinot M, et al. Mol Membr Biol. 2010 Nov;27(8):428-42. doi: 10.3109/09687688.2010.510485. Epub 2010 Nov 11. Mol Membr Biol. 2010. PMID: 21067455 Review.
Cited by
- Solution Asymmetry and Salt Expand Fluid-Fluid Coexistence Regions of Charged Membranes.
Kubsch B, Robinson T, Lipowsky R, Dimova R. Kubsch B, et al. Biophys J. 2016 Jun 21;110(12):2581-2584. doi: 10.1016/j.bpj.2016.05.028. Epub 2016 Jun 7. Biophys J. 2016. PMID: 27288275 Free PMC article. - Regulation of the Golgi complex by phospholipid remodeling enzymes.
Ha KD, Clarke BA, Brown WJ. Ha KD, et al. Biochim Biophys Acta. 2012 Aug;1821(8):1078-88. doi: 10.1016/j.bbalip.2012.04.004. Epub 2012 Apr 22. Biochim Biophys Acta. 2012. PMID: 22562055 Free PMC article. Review. - Amphipathic motifs in BAR domains are essential for membrane curvature sensing.
Bhatia VK, Madsen KL, Bolinger PY, Kunding A, Hedegård P, Gether U, Stamou D. Bhatia VK, et al. EMBO J. 2009 Nov 4;28(21):3303-14. doi: 10.1038/emboj.2009.261. Epub 2009 Oct 8. EMBO J. 2009. PMID: 19816406 Free PMC article. - COPI budding within the Golgi stack.
Popoff V, Adolf F, Brügger B, Wieland F. Popoff V, et al. Cold Spring Harb Perspect Biol. 2011 Nov 1;3(11):a005231. doi: 10.1101/cshperspect.a005231. Cold Spring Harb Perspect Biol. 2011. PMID: 21844168 Free PMC article. Review. - Phase Behavior of Charged Vesicles Under Symmetric and Asymmetric Solution Conditions Monitored with Fluorescence Microscopy.
Kubsch B, Robinson T, Steinkühler J, Dimova R. Kubsch B, et al. J Vis Exp. 2017 Oct 24;(128):56034. doi: 10.3791/56034. J Vis Exp. 2017. PMID: 29155700 Free PMC article.
References
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. - PubMed
- Bethune J, Wieland F, Moelleken J. COPI-mediated transport. J Membr Biol. 2006;211:65–79. - PubMed
- Antonny B, Beraud-Dufour S, Chardin P, Chabre M. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 1997;36:4675–4684. - PubMed
- Ostermann J, et al. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–1025. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources