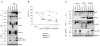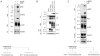The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin - PubMed (original) (raw)
The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin
Laura Silvestri et al. Cell Metab. 2008 Dec.
Abstract
The liver peptide hepcidin regulates body iron, is upregulated in iron overload and inflammation, and is downregulated in iron deficiency/hypoxia. The transmembrane serine protease matriptase-2 (TMPRSS6) inhibits the hepcidin response and its mutational inactivation causes iron-deficient anemia in mice and humans. Here we confirm the inhibitory effect of matriptase-2 on hepcidin promoter; we show that matriptase-2 lacking the serine protease domain, identified in the anemic Mask mouse (matriptase-2(MASK)), is fully inactive and that mutant R774C found in patients with genetic iron deficiency has decreased inhibitory activity. Matriptase-2 cleaves hemojuvelin (HJV), a regulator of hepcidin, on plasma membrane; matriptase-2(MASK) shows no cleavage activity and the human mutant only partial cleavage capacity. Matriptase-2 interacts with HJV through the ectodomain since the interaction is conserved in matriptase-2(MASK). The expression of matriptase-2 mutants in zebrafish results in anemia, confirming the matriptase-2 role in iron metabolism and its interaction with HJV.
Figures
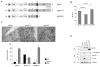
Figure 1. Matriptase-2 variants processing and plasma membrane localization
(A) Schematic representation of matriptase-2 (MT2) functional domains and localization of the studied mutations [modified from (Ramsay et al., 2008)]. TM: transmembrane domain. SEA: sea urchin sperm protein, enteropeptidase agrin. CUB: complement protein subcomponents C1r/C1s, urchin embryonic growth factor and bone morphogenetic protein 1 domain. L: low density lipoprotein receptor class A domain. S/P: serine protease domain. The arrow indicates the cleavage activation site. Asterisks indicate the predicted consensus N-glycosylation sites. (B) Quantification of membrane bound matriptase-2 (m-MT2) by binding assay. Hela cells were transiently transfected with the wild type and mutant expressing vectors, or the empty vector, and analyzed for the amount of matriptase-2 on the cell surface. The amount was calculated as the ratio between the absorbance of unpermeabilized and permeabilized cells. Error bars indicate standard deviation. Statistical significance was calculated on a total of three experiments, made in triplicate. Exact P-values are shown above bars. (C) Electron microscopy and morphometric analysis of MT2wt, MT2R774C and MT2MASK. (D) Characterization of MT2. Whole cell extracts and concentrated media of transiently transfected HeLa cells were analyzed by 10% SDS-PAGE. Western blot was performed following standard procedures; MT2 was revealed by the anti-FLAG antibody. s-MT2: soluble MT2. S/P: serine protease domain. CL: cellular lysates; CM: conditioned medium. The equal loading was verified by α-tubulin.
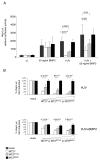
Figure 2. Matriptase-2 modulation of the hepcidin promoter responses
(A) Hepcidin promoter responses by BMP2 and HJV, in the presence of matriptase-2 (MT2). A firefly luciferase reporter driven by 2.9 kb of the proximal hepcidin promoter was cotransfected into Hep3B cells with pRL-TK, either alone or with HJV and/or MT2 expressing vectors. Relative luciferase activity is calculated as reported in material and method and expressed as a multiple of the activity of cells transfected with the reporter alone. (B) Dose dependent modulation of the hepcidin activity by MT2. Hep3B cells were transfected with increasing concentrations of wild type or mutant MT2 expressing vectors in the presence of fixed amount of HJV expressing vector and treated or not with BMP2 (50 ng/ml). The fold induction is calculated as a ratio between the MT2-mediated and the mock-mediated hepcidin promoter activity, calculated as described in (A). Experiments, made in triplicate, were performed three times. Error bars indicate SD. Exact P-values are shown above bars.
Figure 3. Matriptase-2 cleaves membrane HJV
(A) HeLa cells were transfected with HJV in the presence of the empty vector (mock), matriptase-2wt (MT2wt), MT2R774C and MT2MASK. Whole cell extracts, concentrated media and PI-PLC supernatants were loaded onto a 10% SDS-PAGE and processed for western blot analysis. Anti-FLAG and anti-HJV were used to detect MT2 and HJV respectively. β-ME: beta-mercaptoethanol. (B) Binding assay was used to measure m-HJV in the presence of increasing concentrations of wild type and mutants MT2 expressing vectors and was performed essentially as described in Fig. 1B. Experiments were made in triplicate and performed three times. Error bars indicate SD. Exact P-values refer to MT2wt versus MT2R774C. (C) HeLa cells were transfected with MT2wt and MT2MASK, in the presence of HJVwt, HJVW191C and HJVR335Q. Whole cell extracts, concentrated media and PI-PLC supernatants were loaded onto a 10% SDS-PAGE and processed for western blot analyses. s-HJV: soluble HJV; m-HJV: membrane associated HJV. CL: cellular lysates; CM: conditioned medium; PI-PLC: supernatant after PI-PLC cleavage. The equal loading was verified by α-tubulin.
Figure 4. Matriptase-2 does not cleave soluble HJV
(A) HeLa cells were transfected with empty vector or matriptase-2 (MT2) expressing plasmid, and then incubated with an exogenous source of soluble HJV. Whole cell extracts and concentrated media were then analyzed by western blot. A scheme of the experiment is shown bottom left. (B) HeLa cells were transfected with HJV and increasing concentration of MT2wt expressing vectors. Whole cell extracts, concentrated media and PI-PLC derived supernatants were then analyzed by western blot. (C) HeLa cells were transfected with HJV cDNA expressing vector and incubated with exogenous soluble MT2. Whole cell extracts, concentrated media and PI-PLC derived proteins were analyzed by western blot. A scheme of the experiment is shown bottom right. s-HJV and s-MT2 refer to soluble proteins. m-HJV indicates membrane-bound HJV. CL: cellular lysates; CM: conditioned medium; PI-PLC: supernatant after PI-PLC cleavage. The equal loading was verified by α-tubulin.
Figure 5. Matriptase-2 interacts with HJV
HeLa cells were cotransfected with matriptase-2wt (MT2wt)(A) and MT2MASK (B) in the presence of HJV or an empty vector. Precleared whole cell extracts were immunoprecipitated with anti-HJV and revealed with the anti-FLAG antibody, which recognizes MT2 (panels A and B) or immunoprecipitated with anti-FLAG and revealed with anti-HJV (panel A). To control for transfection, whole cell extracts were loaded and revealed with anti-HJV and anti-FLAG antibodies.
Figure 6. Expression of mutants matriptase-2 in zebrafish affects hemoglobinization of erythrocytes
(A) Zebrafish embryos were injected with control (z_control_ MO) and matriptase-2 morpholino (z_mt2_ MO). Fourty eight hours post injection embryos were stained with o_-dianisidine to detect hemoglobinized cells. (B) Embryos as in (A) were homogenized and RNA was extracted as described in Material and Methods. RT-PCR was performed using oligonucleotides specific for matriptase-2 (z_mt2) and for the elongation factor 1α(z_ef1α_). Four hundred fifty embryos were injected and the survival rate was 85%. (C) Zebrafish embryos were injected with matriptase-2wt (MT2wt), MT2R774C and MT2MASK expressing vectors, in the presence (+ Fe) or not (− Fe) of iron dextran. At 48 hours after fertilization embryos were stained with _o_-dianisidine to detect hemoglobinized cells. Representative pictures of the injected fishes (200 for each construct, made in triplicate) are shown. (D) MT2 levels were assayed by western blot analysis.
Figure 7. Model of matriptase-2 activity on BMP-HJV-hepcidin pathway
(A) Schematic representation of a model of matriptase-2 activity in iron deficiency. On the left, the serine protease cleaves m-HJV releasing soluble fragments (here simplified by the black boxes). The cleavage sites of matriptase-2 are unknown. The question mark indicates uncertainty on fragments function. The resulting hepcidin inhibition is shown. The complementary effect of s-HJV, produced by furin cleavage, to sequester BMP is shown on the right. (B) Lack of hepcidin inhibition in the presence of matriptase-2 mutations. m-HJV acts as BMP coreceptor and permits hepcidin production in iron deficiency; the effect of s-HJV cannot downregulate hepcidin in the presence of m-HJV.
Similar articles
- Retinal expression of the serine protease matriptase-2 (Tmprss6) and its role in retinal iron homeostasis.
Gnana-Prakasam JP, Baldowski RB, Ananth S, Martin PM, Smith SB, Ganapathy V. Gnana-Prakasam JP, et al. Mol Vis. 2014 Apr 26;20:561-74. eCollection 2014. Mol Vis. 2014. PMID: 24791141 Free PMC article. - Matriptase-2 and Hemojuvelin in Hepcidin Regulation: In Vivo Immunoblot Studies in Mask Mice.
Krijt J, Frýdlová J, Gurieva I, Přikryl P, Báječný M, Steinbicker AU, Vokurka M, Truksa J. Krijt J, et al. Int J Mol Sci. 2021 Mar 6;22(5):2650. doi: 10.3390/ijms22052650. Int J Mol Sci. 2021. PMID: 33800732 Free PMC article. - Liver hemojuvelin protein levels in mice deficient in matriptase-2 (Tmprss6).
Krijt J, Fujikura Y, Ramsay AJ, Velasco G, Nečas E. Krijt J, et al. Blood Cells Mol Dis. 2011 Aug 15;47(2):133-7. doi: 10.1016/j.bcmd.2011.04.009. Epub 2011 May 25. Blood Cells Mol Dis. 2011. PMID: 21612955 - Into the matrix: regulation of the iron regulatory hormone hepcidin by matriptase-2.
Knutson MD. Knutson MD. Nutr Rev. 2009 May;67(5):284-8. doi: 10.1111/j.1753-4887.2009.00200.x. Nutr Rev. 2009. PMID: 19386032 Review. - Matriptase-2 (TMPRSS6): a proteolytic regulator of iron homeostasis.
Ramsay AJ, Hooper JD, Folgueras AR, Velasco G, López-Otín C. Ramsay AJ, et al. Haematologica. 2009 Jun;94(6):840-9. doi: 10.3324/haematol.2008.001867. Epub 2009 Apr 18. Haematologica. 2009. PMID: 19377077 Free PMC article. Review.
Cited by
- Serum Iron Overload Triggers the SMAD Pathway and Induces Hepcidin Expression in Hepatocytes through SMURF1.
Yazal T, Li CY. Yazal T, et al. J Clin Transl Hepatol. 2024 Sep 28;12(9):761-762. doi: 10.14218/JCTH.2024.00220. Epub 2024 Aug 13. J Clin Transl Hepatol. 2024. PMID: 39280070 Free PMC article. No abstract available. - Whole genome discovery of regulatory genes responsible for the response of chicken to heat stress.
Hosseinzadeh S, Hasanpur K. Hosseinzadeh S, et al. Sci Rep. 2024 Mar 19;14(1):6544. doi: 10.1038/s41598-024-56757-0. Sci Rep. 2024. PMID: 38503864 Free PMC article. - Oral iron therapy: Current concepts and future prospects for improving efficacy and outcomes.
Ebea-Ugwuanyi PO, Vidyasagar S, Connor JR, Frazer DM, Knutson MD, Collins JF. Ebea-Ugwuanyi PO, et al. Br J Haematol. 2024 Mar;204(3):759-773. doi: 10.1111/bjh.19268. Epub 2024 Jan 22. Br J Haematol. 2024. PMID: 38253961 Review. - Activation of Intestinal HIF2α Ameliorates Iron-Refractory Anemia.
Yu Y, Su Y, Yang S, Liu Y, Lin Z, Das NK, Wu Q, Zhou J, Sun S, Li X, Yue W, Shah YM, Min J, Wang F. Yu Y, et al. Adv Sci (Weinh). 2024 Mar;11(12):e2307022. doi: 10.1002/advs.202307022. Epub 2024 Jan 20. Adv Sci (Weinh). 2024. PMID: 38243847 Free PMC article. - Genetically determined circulating micronutrients and the risk of nonalcoholic fatty liver disease.
Liu K, Chen Y, Chen J, Chen W, Sun X, Mao Y, Ye D. Liu K, et al. Sci Rep. 2024 Jan 11;14(1):1105. doi: 10.1038/s41598-024-51609-3. Sci Rep. 2024. PMID: 38212362 Free PMC article.
References
- Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nature genetics. 2006;38:531–539. - PubMed
- Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Ben Amitai D, Lurie R, Pasmanik-Chor M, Indelman M, Zvulunov A, Saban S, Magal N, Sprecher E, Shohat M. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. American journal of human genetics. 2007;80:467–477. - PMC - PubMed
- Bernascone I, Vavassori S, Di Pentima A, Santambrogio S, Lamorte G, Amoroso A, Scolari F, Ghiggeri GM, Casari G, Polishchuk R, Rampoldi L. Defective intracellular trafficking of uromodulin mutant isoforms. Traffic (Copenhagen, Denmark) 2006;7:1567–1579. - PubMed
- Camaschella C. Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders. Blood. 2005;106:3710–3717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases