Inactivation of the CYLD deubiquitinase by HPV E6 mediates hypoxia-induced NF-kappaB activation - PubMed (original) (raw)
Inactivation of the CYLD deubiquitinase by HPV E6 mediates hypoxia-induced NF-kappaB activation
Jiabin An et al. Cancer Cell. 2008.
Abstract
The biochemical mechanisms that underlie hypoxia-induced NF-kappaB activity have remained largely undefined. Here, we find that prolonged hypoxia-induced NF-kappaB activation is restricted to cancer cell lines infected with high-risk human papillomavirus (HPV) serotypes. The HPV-encoded E6 protein is necessary and sufficient for prolonged hypoxia-induced NF-kappaB activation in these systems. The molecular target of E6 in the NF-kappaB pathway is the CYLD lysine 63 (K63) deubiquitinase, a negative regulator of the NF-kappaB pathway. Specifically, hypoxia stimulates E6-mediated ubiquitination and proteasomal degradation of CYLD. Given the established role of NF-kappaB in human carcinogenesis, these findings provide a potential molecular/viral link between hypoxia and the adverse clinical outcomes observed in HPV-associated malignancies.
Figures
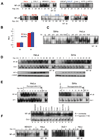
Figure 1. Effects of hypoxia and reoxygenation on NF-κB activation
A. Representative EMSAs for measurement of NF-κB activity under normoxic (21% 02) and hypoxic (1% 02) conditions for the indicated times. WT and M = cold-competition with 100-fold molar excess of wild-type and mutant NF-κB DNA response elements, respectively. Red = HPV-positive squamous cell carcinoma; blue = HPV-negative squamous cell carcinoma; black = non-squamous cell carcinoma. B. NF-κB reporter assays performed 48 hrs after transfection. N = normoxia; H = hypoxia. Results are averages of three experiments ± standard deviation (s.d.). Firefly luciferase expression was normalized to that of Renilla luciferase (see Methods). C. Electrophoretic mobility supershift assays were performed with the indicated antibodies after cells were exposed to hypoxia for 24 hours. D. Top panels: EMSAs demonstrate detailed time course of hypoxia-induced NF-κB activity. Second row: Oct-1 EMSAs serves as a control. Third row: HIF1α Western blots confirm exposure to hypoxia. Bottom row: γ-tubulin Westerns as loading controls. E. Effects of reoxygenation on NF-κB activity (measured by EMSA). EMSAs for AP1 or Oct-1 served as controls. Cells were exposed to hypoxia for 24 hrs followed by return to normoxic conditions (reoxygenation) for the indicated times. F. Transient hypoxia-induced NF-κB activation in HPV-negative cancer cells. Top panels: Shorter course hypoxia exposure (1 hr) of HPV-negative cancer cells. Bottom panels: detailed time course of transient NF-κB activation.
Figure 2. HPV E6 is necessary and sufficient for prolonged hypoxia-induced NF-κB activation
A. Western blots for p53, E7 and pRB (a surrogate for E7 expression). Cells were transfected with the indicated siRNAs and immunoblotting was performed with the indicated antibodies after 48 hours. C = scrambled control siRNA. B. EMSAs show the effects of E6 and E7 RNAi on NF-κB activity. C. Same as B, but NF-κB activity measured by reporter assay performed under hypoxic conditions. Results were normalized to that of the C siRNA. D. NF-κB reporter activity due to transfection of HPV16 E6 under hypoxic conditions. Cells were exposed to normoxia or hypoxia for 48 hours and results are represented as the ratio of NF-κB reporter activity in hypoxia:normoxia. E. NF-κB reporter activity resulting from transfection of E6 plus increasing concentrations of E7. Results calculated as in D. F. NF-κB reporter activity resulting from transfection of HPV16 E6 or E7. G. NF-κB reporter activity resulting from transfection of E6 plus increasing amounts of E7. Results for C through G are averages of three experiments ± s.d. In D through G, the results were normalized to that of the vector controls, and firefly luciferase expression was normalized to that of Renilla luciferase (see Methods). H. EMSAs in primary murine keratinocytes stably transduced with control, E6 or E7 retroviral vectors. I. IKKβ in vitro kinase assays (top two panels) were performed on extracts exposed to normoxia or hypoxia. Successful silencing of E6 is shown in the bottom two panels.
Figure 3. Hypoxia induces TRAF6 ubiquitination in an E6-dependent fashion
A. Cells were co-transfected with the TRAF6-DN or a control expression plasmid plus the NF-κB luciferase reporter. Results are means of three experiments ± s.d. and are represented as the ratio of NF-κB reporter activity in hypoxia:normoxia (48 hrs). Firefly luciferase expression was normalized to that of Renilla luciferase (see Methods). B. Hypoxia-induced TRAF6 polyubiquitination. TRAF6 immunoprecipitates were immunoblotted with ubiquitin (top) or TRAF6 (bottom) antibodies. C. TRAF6 polyubiquitination in normoxia and hypoxia (1 hr). D. TRAF6 polyubiquitination was assessed as in B but after transfection of control or E6 siRNA. All immunoprecipitations were performed under denaturing conditions.
Figure 4. E6-mediated activation of NF-κB is dependent upon CYLD
A. Western blots for CYLD in cells transfected with CYLD or C siRNA. B. Effects of CYLD RNAi on hypoxia-induced NF-κB reporter activity. Results are reported as the ratio of NF-κB activity in hypoxia to normoxia. C. Cells were transfected with an HPV16 E6 expression vector and control or E6-specific siRNA. Protein was harvested at 48 hrs. Western blots for CYLD and actin demonstrate effective silencing of CYLD. D. Effects of CYLD RNAi on hypoxia-induced NF-κB reporter activity. Results are reported as the ratio of NF-κB activity in hypoxia:normoxia. E. Effects of CYLD RNA interference on hypoxia-induced NF-κB activity as measured by EMSA. Top panel: NF-κB EMSA. Bottom panel: Oct-1 EMSA. F. Ratio of NF-κB reporter activity (hypoxia:normoxia) in primary wild-type (CYLD+/+) or CYLD “knock-out” (_CYLD_−/−) primary epidermal keratinocytes transfected with a HPV16E6 or vector control plasmid. All reporter results are averages of three experiments ± s.d. Duration of hypoxia was 24 hours. G. Effects of E6 expression on NF-κB activation in CYLD+/+ or _CYLD_−/− primary epidermal keratinocytes assayed by EMSA. C = lentiviral control transduction. E6 = HA-E6 lentiviral transduction. N = normoxia; H = hypoxia for 12 hours.
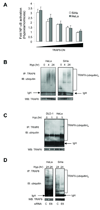
Figure 5. E6 reduces CYLD expression through a proteasome-dependent mechanism
A. Western blots for CYLD expression in cells transfected with E6 or control siRNA. B. CYLD expression in cells transfected with combinations of HPV16 E6 or Flag-tagged CYLD. C. Same as B except cells were treated with the proteasome inhibitor, MG132 (10 µM), two hrs prior to harvesting protein. D. Ubiquitination of CYLD in the presence of E6. Cells were exposed to MG132 (10 µM) for two hrs prior to harvesting protein. IP = immunoprecipitation; IB = immunoblot. Ubiquitination assays were performed under denaturing conditions.
Figure 6. Effects of hypoxia and hydroxylation inhibition on CYLD expression and ubiquitination
A. Top and middle panels: Western blots for CYLD and actin after cells were exposed to hypoxia for the indicated times. Bottom panel: Same as top panels, but cells were treated with MG132 for 2 hours prior to protein extraction. B. CYLD and actin Western blots on protein harvested from the indicated HPV-negative cells exposed to hypoxia. C. CYLD ubiquitination status under normoxia, hypoxia, and upon inhibition of hydroxylase activity with DMOG. Cells were exposed to hypoxia or DMOG for six hrs in the presence of MG132 (10µM) to prevent CYLD degradation. Protein was immunoprecipitated with an anti-CYLD antibody (or IgG isotype control) and then subjected to immunoblotting with the indicated antibodies. The bottom panels demonstrate equivalent input of CYLD into all lanes. D. Effects of E6 gene silencing on hypoxia-induced CYLD ubiquitination. Cells were transfected with E6-specific or control siRNA and, after 24 hrs, exposed to normoxia or hypoxia for an additional 6 hrs. Co-immunoprecipitation to assess CYLD ubiquitination was performed as in C. The bottom panels demonstrated effective silencing of E6 protein expression. (Current antibodies cannot detect E6 by direct immunoblotting, so E6 immunoprecipitation followed by E6 immunoblotting with two different E6 antibodies was performed).
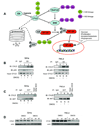
Figure 7. The E6-CYLD protein-protein interaction is enhanced by hypoxia and hydroxylase inhibition
A. Schematic diagram depicting the mechanism whereby HPVencoded E6 activates NF-κB through ubiquitination and subsequent proteasomal degradation of CYLD. B. Protein extracts (same extracts as Figure 6C) were exposed to normoxia, hypoxia or DMOG for six hrs in the presence of MG132 (10 µM) and then subjected to co-immunoprecipitation studies to evaluate E6-CYLD protein-protein interactions (top panels). E6-p53 co-immunoprecipitations (bottom panels). Immunoprecipitations with an isotype control antibody (lanes 1 and 5). C. CYLD does not immunoprecipitate with AKT. Experiments were performed as in B with indicated antibodies. For B and C, the protein extracts were the same as those employed for Figure 6. D. CYLD protein expression was assessed cells treated with DMOG or vehicle (DMSO).
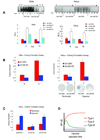
Figure 8. Hypoxia-induced enhancement of anchorage-independent growth is NF-κB dependent
A. EMSAs and reporter assays to define the multiplicity of infection (moi) of the Ad-IB-SR that blocks hypoxia-induced NF-κB activity. The EMSAs were performed after 24 hrs of hypoxia exposure, and reporter activity was assayed after 48 hrs of hypoxia. The reporter results are the means of three experiments ± s.d.. B. Results of anchorage-independent growth assays (at 14 days) were normalized to that of the normoxic group transduced with the control virus (Ad-CMV). Representative phase-contrast photomicrographs are shown (final magnification = 200X). C. Anchorage-independent growth of HeLa cells transfected with the indicated plasmids (CYLD, C/S-CYLD or vector control) under G418 selection. D. Model of cellular NF-κB responses to hypoxia (see Discussion for details).
Similar articles
- Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes.
Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. Nees M, et al. J Virol. 2001 May;75(9):4283-96. doi: 10.1128/JVI.75.9.4283-4296.2001. J Virol. 2001. PMID: 11287578 Free PMC article. - CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes.
Sun SC. Sun SC. Cell Death Differ. 2010 Jan;17(1):25-34. doi: 10.1038/cdd.2009.43. Cell Death Differ. 2010. PMID: 19373246 Free PMC article. Review. - Activation of telomerase by HPVs.
Katzenellenbogen RA. Katzenellenbogen RA. Virus Res. 2017 Mar 2;231:50-55. doi: 10.1016/j.virusres.2016.11.003. Epub 2016 Nov 15. Virus Res. 2017. PMID: 27863966 Review.
Cited by
- The Hallmarks of Cervical Cancer: Molecular Mechanisms Induced by Human Papillomavirus.
Rosendo-Chalma P, Antonio-Véjar V, Ortiz Tejedor JG, Ortiz Segarra J, Vega Crespo B, Bigoni-Ordóñez GD. Rosendo-Chalma P, et al. Biology (Basel). 2024 Jan 27;13(2):77. doi: 10.3390/biology13020077. Biology (Basel). 2024. PMID: 38392296 Free PMC article. Review. - Manipulation of the innate immune response by human papillomaviruses.
Hong S, Laimins LA. Hong S, et al. Virus Res. 2017 Mar 2;231:34-40. doi: 10.1016/j.virusres.2016.11.004. Epub 2016 Nov 5. Virus Res. 2017. PMID: 27826042 Free PMC article. Review. - The Role of Deubiquitinases in Oncovirus and Host Interactions.
Li Y, Shi F, Hu J, Xie L, Bode AM, Cao Y. Li Y, et al. J Oncol. 2019 Jul 18;2019:2128410. doi: 10.1155/2019/2128410. eCollection 2019. J Oncol. 2019. PMID: 31396277 Free PMC article. Review. - Viral oncogenes, noncoding RNAs, and RNA splicing in human tumor viruses.
Zheng ZM. Zheng ZM. Int J Biol Sci. 2010 Dec 1;6(7):730-55. doi: 10.7150/ijbs.6.730. Int J Biol Sci. 2010. PMID: 21152115 Free PMC article. Review. - CYLD in health and disease.
Marín-Rubio JL, Raote I, Inns J, Dobson-Stone C, Rajan N. Marín-Rubio JL, et al. Dis Model Mech. 2023 Jun 1;16(6):dmm050093. doi: 10.1242/dmm.050093. Epub 2023 Jun 30. Dis Model Mech. 2023. PMID: 37387450 Free PMC article. Review.
References
- Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. - PubMed
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. - PubMed
- Butz K, Ristriani T, Hengstermann A, Denk C, Scheffner M, Hoppe-Seyler F. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene. 2003;22:5938–5945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases