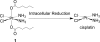Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles - PubMed (original) (raw)
Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles
Shanta Dhar et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Cisplatin is used to treat a variety of tumors, but dose limiting toxicities or intrinsic and acquired resistance limit its application in many types of cancer including prostate. We report a unique strategy to deliver cisplatin to prostate cancer cells by constructing Pt(IV)-encapsulated prostate-specific membrane antigen (PSMA) targeted nanoparticles (NPs) of poly(D,L-lactic-co-glycolic acid) (PLGA)-poly(ethylene glycol) (PEG)-functionalized controlled release polymers. By using PLGA-b-PEG nanoparticles with PSMA targeting aptamers (Apt) on the surface as a vehicle for the platinum(IV) compound c,t,c-[Pt(NH(3))(2)(O(2)CCH(2)CH(2)CH(2)CH(2)CH(3))(2)Cl(2)] (1), a lethal dose of cisplatin was delivered specifically to prostate cancer cells. PSMA aptamer targeted delivery of Pt(IV) cargos to PSMA(+) LNCaP prostate cancer cells by endocytosis of the nanoparticle vehicles was demonstrated using fluorescence microscopy by colocalization of green fluorescent labeled cholesterol-encapsulated NPs and early endosome marker EEA-1. The choice of linear hexyl chains in 1 was the result of a systematic study to optimize encapsulation and controlled release from the polymer without compromising either feature. Release of cisplatin from the polymeric nanoparticles after reduction of 1 and formation of cisplatin 1,2-intrastrand d(GpG) cross-links on nuclear DNA was confirmed by using a monoclonal antibody for the adduct. A comparison between the cytotoxic activities of Pt(IV)-encapsulated PLGA-b-PEG NPs with the PSMA aptamer on the surface (Pt-NP-Apt), cisplatin, and the nontargeted Pt(IV)-encapsulated NPs (Pt-NP) against human prostate PSMA-overexpressing LNCaP and PSMA(-) PC3 cancer cells revealed significant differences. The effectiveness of PSMA targeted Pt-NP-Apt nanoparticles against the PSMA(+) LNCaP cells is approximately an order of magnitude greater than that of free cisplatin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Scheme 1.
Chemical structure of the hydrophobic platinum(IV) compound 1 and the chemistry by which the active drug, cisplatin is released, after reduction in the cell.
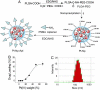
Fig. 1.
Construction and properties of aptamer-functionalized Pt(IV) nanoparticles. (A) Synthesis of Pt(IV)-encapsulated PLGA-_b_-PEG-COOH nanoparticles by nanoprecipitation and conjugation of PSMA aptamer to NP. (B) Loading of 1 in the PLGA-_b_-PEG-COOH nanoparticles. (C) Size of the Pt(IV)-encapsulated nanoparticles.
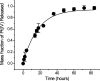
Fig. 2.
In vitro release kinetics of encapsulated Pt(IV) compound 1 from PLGA-_b_-PEG nanoparticles in PBS (pH 7.4) at 37 °C.
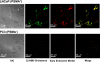
Fig. 3.
Detection of endosome formation and cellular uptake of Pt-NP-Apt in LNCaP cells by fluorescence microscopy. Green fluorescent 22-NBD-cholesterol and 1 were encapsulated in the PLGA-_b_-PEG nanoparticles and PSMA aptamers were conjugated to the surface of the particles. The early endosomes were visualized in red by using the early endosome marker EEA-1.
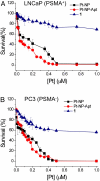
Fig. 4.
Cytotoxicity profiles of PSMA aptamer-targeted Pt(IV)-encapsulated PLGA-_b_-PEG nanoparticles (Pt-NP-Apt) (red circles), nontargeted nanoparticles (Pt-NP) (black squares), and compound 1 (blue triangles) with (A) PSMA+ LNCaP cells and (B) PSMA− PC3 cells after 72 h as determined by the MTT assay.
Similar articles
- Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo.
Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Dhar S, et al. Proc Natl Acad Sci U S A. 2011 Feb 1;108(5):1850-5. doi: 10.1073/pnas.1011379108. Epub 2011 Jan 13. Proc Natl Acad Sci U S A. 2011. PMID: 21233423 Free PMC article. - Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy.
Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, Langer R, Farokhzad OC. Kolishetti N, et al. Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):17939-44. doi: 10.1073/pnas.1011368107. Epub 2010 Oct 4. Proc Natl Acad Sci U S A. 2010. PMID: 20921363 Free PMC article. - Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery.
Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R, Farokhzad OC. Cheng J, et al. Biomaterials. 2007 Feb;28(5):869-76. doi: 10.1016/j.biomaterials.2006.09.047. Epub 2006 Oct 20. Biomaterials. 2007. PMID: 17055572 Free PMC article. - Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.
Hadinoto K, Sundaresan A, Cheow WS. Hadinoto K, et al. Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review. - Thermally cross-linked superparamagnetic iron oxide nanoparticle-A10 RNA aptamer-doxorubicin conjugate.
Zhang H. Zhang H. 2008 Aug 29 [updated 2008 Oct 8]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Aug 29 [updated 2008 Oct 8]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641568 Free Books & Documents. Review.
Cited by
- Biomolecule-functionalized nanoformulations for prostate cancer theranostics.
Pranav, Laskar P, Jaggi M, Chauhan SC, Yallapu MM. Pranav, et al. J Adv Res. 2023 Sep;51:197-217. doi: 10.1016/j.jare.2022.11.001. Epub 2022 Nov 9. J Adv Res. 2023. PMID: 36368516 Free PMC article. Review. - Aptamer-hybrid nanoparticle bioconjugate efficiently delivers miRNA-29b to non-small-cell lung cancer cells and inhibits growth by downregulating essential oncoproteins.
Perepelyuk M, Maher C, Lakshmikuttyamma A, Shoyele SA. Perepelyuk M, et al. Int J Nanomedicine. 2016 Jul 28;11:3533-44. doi: 10.2147/IJN.S110488. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27555773 Free PMC article. - Tat peptide-decorated gelatin-siloxane nanoparticles for delivery of CGRP transgene in treatment of cerebral vasospasm.
Tian XH, Wang ZG, Meng H, Wang YH, Feng W, Wei F, Huang ZC, Lin XN, Ren L. Tian XH, et al. Int J Nanomedicine. 2013;8:865-76. doi: 10.2147/IJN.S39951. Epub 2013 Mar 27. Int J Nanomedicine. 2013. PMID: 23576867 Free PMC article. - Development of a Smart Scaffold for Sequential Cancer Chemotherapy and Tissue Engineering.
Sengupta P, Agrawal V, Prasad BLV. Sengupta P, et al. ACS Omega. 2020 Aug 11;5(33):20724-20733. doi: 10.1021/acsomega.9b03694. eCollection 2020 Aug 25. ACS Omega. 2020. PMID: 32875205 Free PMC article. - A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics.
Steichen SD, Caldorera-Moore M, Peppas NA. Steichen SD, et al. Eur J Pharm Sci. 2013 Feb 14;48(3):416-27. doi: 10.1016/j.ejps.2012.12.006. Epub 2012 Dec 20. Eur J Pharm Sci. 2013. PMID: 23262059 Free PMC article. Review.
References
- Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
- Israeli RS, Powell CT, Fair WR, Heston WDW. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–230. - PubMed
- Murphy GP, et al. Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer. 1998;83:2259–2269. - PubMed
- Kawakami M, Nakayama J. Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res. 1997;57:2321–2324. - PubMed
- Wright GL, Jr, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA119349/CA/NCI NIH HHS/United States
- R37 CA034992/CA/NCI NIH HHS/United States
- CA34992/CA/NCI NIH HHS/United States
- K08 EB003647/EB/NIBIB NIH HHS/United States
- EB003647/EB/NIBIB NIH HHS/United States
- U54 CA119349/CA/NCI NIH HHS/United States
- R01 CA034992/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous