Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells - PubMed (original) (raw)
Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells
Esther Hulleman et al. Blood. 2009.
Abstract
Treatment failure in pediatric acute lymphoblastic leukemia (ALL) is related to cellular resistance to glucocorticoids (eg, prednisolone). Recently, we demonstrated that genes associated with glucose metabolism are differentially expressed between prednisolone-sensitive and prednisolone-resistant precursor B-lineage leukemic patients. Here, we show that prednisolone resistance is associated with increased glucose consumption and that inhibition of glycolysis sensitizes prednisolone-resistant ALL cell lines to glucocorticoids. Treatment of prednisolone-resistant Jurkat and Molt4 cells with 2-deoxy-D-glucose (2-DG), lonidamine (LND), or 3-bromopyruvate (3-BrPA) increased the in vitro sensitivity to glucocorticoids, while treatment of the prednisolone-sensitive cell lines Tom-1 and RS4; 11 did not influence drug cytotoxicity. This sensitizing effect of the glycolysis inhibitors in glucocorticoid-resistant ALL cells was not found for other classes of antileukemic drugs (ie, vincristine and daunorubicin). Moreover, down-regulation of the expression of GAPDH by RNA interference also sensitized to prednisolone, comparable with treatment with glycolytic inhibitors. Importantly, the ability of 2-DG to reverse glucocorticoid resistance was not limited to cell lines, but was also observed in isolated primary ALL cells from patients. Together, these findings indicate the importance of the glycolytic pathway in glucocorticoid resistance in ALL and suggest that targeting glycolysis is a viable strategy for modulating prednisolone resistance in ALL.
Figures
Figure 1
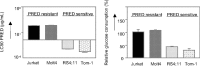
Glycolysis is up-regulated in prednisolone-resistant human leukemia cells. Graphic representation of in vitro prednisolone responsiveness (left panel) and glucose consumption (right panel) of 2 prednisolone-resistant and 2 prednisolone-sensitive human ALL cell lines. Response to prednisolone was measured by the MTT assay; glucose consumption was calculated per cell by measuring the conversion of glucose to 6-phosphogluconate. The glucose consumption in Jurkat cells after 4 days of incubation was set to be 100%, corresponding to approximately 75% of the total glucose present in the medium (1.5 g/L). A representative experiment is shown; data are presented as means plus or minus SD (n = 3).
Figure 2
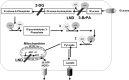
Schematic representation of glucose metabolism in mammalian cells. Glycolytic inhibitors used in this study are indicated as 2-DG, 3-BrPA, and LND.
Figure 3
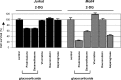
Effect of 2-DG treatment on glucose consumption and prednisolone- induced cytotoxicity in human ALL cell lines. Graphic representation of relative glucose consumption (A) or in vitro prednisolone responsiveness (B) in 2 prednisolone-resistant and 2 prednisolone-sensitive ALL cell lines after 2-DG treatment. Glucose consumption was calculated by measuring the conversion of glucose to 6-phosphogluconate, and glucose consumption in Jurkat cells was set at 100%. Response to prednisolone was measured by the MTT assay; cell survival in nontreated cells was set at 100%. Used concentrations of prednisolone and 2-DG varied depending on cellular toxicity (550 μg/mL and 0.078 μg/mL prednisolone, and 2 mM and 0.5 mM for -resistant and -sensitive cell lines, respectively). Representative experiments are shown; data are presented as means plus or minus SD (n = 3).
Figure 4
Modulation of drug-resistance by 2-DG. Graphic representation of in vitro responsiveness to cytotoxic drugs in 2 prednisolone-resistant ALL cell lines after 2-DG treatment, as assessed by the MTT assay. Concentrations used were 1 mM (Molt4) or 2 mM 2-DG (Jurkat), 550 μg/mL prednisolone, 100 μg/mL dexamethasone, 0.5 ng/mL vincristine, and 0.0098 U/mL L-asparaginase. Cell survival in cells treated only with 2-DG was set at 100% to visualize the effect of synergy. Representative experiments are shown; data are presented as means plus or minus SD (n = 3).
Figure 5
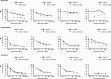
Effect of glycolytic inhibitors on prednisolone-induced cytotoxicity in prednisolone–resistant and prednisolone-sensitive cell lines. Cell survival curves representing in vitro prednisolone responsiveness, as assessed by the MTT assay in 2 prednisolone-resistant (top panel) and 2 prednisolone-sensitive ALL cell lines (bottom panel) after treatment with glycolytic inhibitors. Cells were treated with 1 mM (Jurkat, Molt4) or 0.5 mM 2-DG (▲), 62.5 μM LND ( ), 30 μM 3-BrPA (●) in combination with prednisolone, as indicated in the figure. Cells treated only with prednisolone served as controls (■). To visualize synergy, the survival rate of prednisolone in combination with an inhibitor was corrected for the toxicity caused by the inhibitor itself (set to 100%). A representative experiment is shown; data are presented as means plus or minus SD (n = 3).
), 30 μM 3-BrPA (●) in combination with prednisolone, as indicated in the figure. Cells treated only with prednisolone served as controls (■). To visualize synergy, the survival rate of prednisolone in combination with an inhibitor was corrected for the toxicity caused by the inhibitor itself (set to 100%). A representative experiment is shown; data are presented as means plus or minus SD (n = 3).
Figure 6
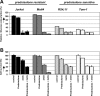
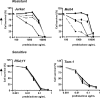
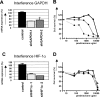
Inhibition of GAPDH expression by RNA interference increases prednisolone-induced cytotoxicity. (A) mRNA levels of GAPDH in Jurkat cells as measured by qPCR. Two different shRNA sequences targeting GAPDH were used (shGAPDH-1 and -2). Expression of cells infected with nonsilencing shRNA sequences was set at 100% and relative expression levels were calculated. (B) Cell survival curves representing in vitro prednisolone responsiveness after RNA interference in cells interfered for GAPDH ( , ▲) or in cells infected with a nonsilencing shRNA sequence (■). (C) mRNA levels of HIF-1α in Jurkat cells as measured by qPCR. Two different shRNA sequences targeting HIF-1α were used (sh HIF-1α-1 and -2). Expression of cells infected with nonsilencing shRNA sequences was set at 100%, and relative expression levels were calculated. (D) Cell survival curves representing in vitro prednisolone responsiveness after RNA interference in cells interfered for HIF-1α (
, ▲) or in cells infected with a nonsilencing shRNA sequence (■). (C) mRNA levels of HIF-1α in Jurkat cells as measured by qPCR. Two different shRNA sequences targeting HIF-1α were used (sh HIF-1α-1 and -2). Expression of cells infected with nonsilencing shRNA sequences was set at 100%, and relative expression levels were calculated. (D) Cell survival curves representing in vitro prednisolone responsiveness after RNA interference in cells interfered for HIF-1α ( , ▲) or in cells infected with a nonsilencing shRNA sequence (■). A representative experiment is shown; data are presented as means plus or minus SD (n = 3).
, ▲) or in cells infected with a nonsilencing shRNA sequence (■). A representative experiment is shown; data are presented as means plus or minus SD (n = 3).
Figure 7
Effect of 2-DG treatment on prednisolone-induced cytotoxicity in primary ALL cells. Cell survival curves representing in vitro prednisolone responsiveness, in prednisolone-resistant ALL patients (top panel), -intermediate patients (middle panel), and -sensitive patients (bottom panel) as assessed by the MTT assay. Concentrations 2-DG varied from 0.2 mM to 2 mM (▼), depending on the patient sample sensitivity. To visualize the synergy, the survival of cells treated with prednisolone and 2-DG was corrected for the toxic effect of 2-DG given as single drug (set at 100%). Cells treated only with prednisolone served as controls (■). A representative experiment is shown; data are presented as means plus or minus SD (n = 3).
Similar articles
- The synergism of MCL1 and glycolysis on pediatric acute lymphoblastic leukemia cell survival and prednisolone resistance.
Ariës IM, Hansen BR, Koch T, van den Dungen R, Evans WE, Pieters R, den Boer ML. Ariës IM, et al. Haematologica. 2013 Dec;98(12):1905-11. doi: 10.3324/haematol.2013.093823. Epub 2013 Oct 18. Haematologica. 2013. PMID: 24142999 Free PMC article. - Genomewide identification of prednisolone-responsive genes in acute lymphoblastic leukemia cells.
Tissing WJ, den Boer ML, Meijerink JP, Menezes RX, Swagemakers S, van der Spek PJ, Sallan SE, Armstrong SA, Pieters R. Tissing WJ, et al. Blood. 2007 May 1;109(9):3929-35. doi: 10.1182/blood-2006-11-056366. Epub 2007 Jan 11. Blood. 2007. PMID: 17218380 Clinical Trial. - The SWI/SNF chromatin-remodeling complex and glucocorticoid resistance in acute lymphoblastic leukemia.
Pottier N, Yang W, Assem M, Panetta JC, Pei D, Paugh SW, Cheng C, Den Boer ML, Relling MV, Pieters R, Evans WE, Cheok MH. Pottier N, et al. J Natl Cancer Inst. 2008 Dec 17;100(24):1792-803. doi: 10.1093/jnci/djn416. Epub 2008 Dec 9. J Natl Cancer Inst. 2008. PMID: 19066270 Free PMC article. - Gene Expression and Resistance to Glucocorticoid-Induced Apoptosis in Acute Lymphoblastic Leukemia: A Brief Review and Update.
Lambrou GI, Adamaki M, Hatziagapiou K, Vlahopoulos S. Lambrou GI, et al. Curr Drug Res Rev. 2020;12(2):131-149. doi: 10.2174/2589977512666200220122650. Curr Drug Res Rev. 2020. PMID: 32077838 Review. - Stable isotope tracing reveals glucose metabolism characteristics of drug-resistant B-cell acute lymphoblastic leukemia.
Hu R, Duan Z, Wang M, Liu M, Zhang Y, Lu Y, Qian Y, Wei E, Feng J, Guo P, Chen Y. Hu R, et al. Anal Chim Acta. 2025 May 22;1352:343884. doi: 10.1016/j.aca.2025.343884. Epub 2025 Mar 3. Anal Chim Acta. 2025. PMID: 40210293 Review.
Cited by
- Targeting cellular metabolism to improve cancer therapeutics.
Zhao Y, Butler EB, Tan M. Zhao Y, et al. Cell Death Dis. 2013 Mar 7;4(3):e532. doi: 10.1038/cddis.2013.60. Cell Death Dis. 2013. PMID: 23470539 Free PMC article. Review. - Mitochondrial control of caspase-dependent and -independent cell death.
Pradelli LA, Bénéteau M, Ricci JE. Pradelli LA, et al. Cell Mol Life Sci. 2010 May;67(10):1589-97. doi: 10.1007/s00018-010-0285-y. Epub 2010 Feb 12. Cell Mol Life Sci. 2010. PMID: 20151314 Free PMC article. Review. - Reversal of glucocorticoid resistance in paediatric acute lymphoblastic leukaemia is dependent on restoring BIM expression.
Toscan CE, Jing D, Mayoh C, Lock RB. Toscan CE, et al. Br J Cancer. 2020 Jun;122(12):1769-1781. doi: 10.1038/s41416-020-0824-8. Epub 2020 Apr 3. Br J Cancer. 2020. PMID: 32242100 Free PMC article. - 2-Deoxy-D-glucose Restore Glucocorticoid Sensitivity in Acute Lymphoblastic Leukemia via Modification of N-Linked Glycosylation in an Oxygen Tension-Independent Manner.
Leni Z, Ćwiek P, Dimitrova V, Dulcey AS, Zamboni N, Simillion C, Rossi G, Leibundgut K, Arcaro A. Leni Z, et al. Oxid Med Cell Longev. 2017;2017:2487297. doi: 10.1155/2017/2487297. Epub 2017 Jul 26. Oxid Med Cell Longev. 2017. PMID: 28814986 Free PMC article. - The anticancer agent 3-bromopyruvate: a simple but powerful molecule taken from the lab to the bedside.
Azevedo-Silva J, Queirós O, Baltazar F, Ułaszewski S, Goffeau A, Ko YH, Pedersen PL, Preto A, Casal M. Azevedo-Silva J, et al. J Bioenerg Biomembr. 2016 Aug;48(4):349-62. doi: 10.1007/s10863-016-9670-z. Epub 2016 Jul 25. J Bioenerg Biomembr. 2016. PMID: 27457582 Review.
References
- Tissing WJ, Meijerink JP, den Boer ML, Pieters R. Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia. 2003;17:17–25. - PubMed
- Ausserlechner MJ, Obexer P, Böck G, Geley S, Kofler R. Cyclin D3 and c-MYC control glucocorticoid-induced cell cycle arrest but not apoptosis in lymphoblastic leukemia cells. Cell Death Differ. 2004;11:165–174. - PubMed
- Ploner C, Schmidt A, Presul E, et al. Glucocorticoid-induced apoptosis and glucocorticoid resistance in acute lymphoblastic leukemia. J Steroid Biochem Mol Biol. 2005;93:153–160. - PubMed
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. - PubMed
- Pieters R, Klumper E, Kaspers GJ, Veerman AJ. Everything you always wanted to know about cellular drug resistance in childhood acute lymphoblastic leukemia. Crit Rev Oncol Hematol. 1997;25:11–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials