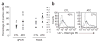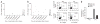Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma - PubMed (original) (raw)
. 2008 Nov;14(11):1264-70.
doi: 10.1038/nm.1882. Epub 2008 Nov 2.
Barbara Savoldo, G Doug Myers, Claudia Rossig, Heidi V Russell, Gianpietro Dotti, M Helen Huls, Enli Liu, Adrian P Gee, Zhuyong Mei, Eric Yvon, Heidi L Weiss, Hao Liu, Cliona M Rooney, Helen E Heslop, Malcolm K Brenner
Affiliations
- PMID: 18978797
- PMCID: PMC2749734
- DOI: 10.1038/nm.1882
Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma
Martin A Pule et al. Nat Med. 2008 Nov.
Abstract
Cytotoxic T lymphocytes (CTLs) directed to nonviral tumor-associated antigens do not survive long term and have limited antitumor activity in vivo, in part because such tumor cells typically lack the appropriate costimulatory molecules. We therefore engineered Epstein-Barr virus (EBV)-specific CTLs to express a chimeric antigen receptor directed to the diasialoganglioside GD2, a nonviral tumor-associated antigen expressed by human neuroblastoma cells. We reasoned that these genetically engineered lymphocytes would receive optimal costimulation after engagement of their native receptors, enhancing survival and antitumor activity mediated through their chimeric receptors. Here we show in individuals with neuroblastoma that EBV-specific CTLs expressing a chimeric GD2-specific receptor indeed survive longer than T cells activated by the CD3-specific antibody OKT3 and expressing the same chimeric receptor but lacking virus specificity. Infusion of these genetically modified cells seemed safe and was associated with tumor regression or necrosis in half of the subjects tested. Hence, virus-specific CTLs can be modified to function as tumor-directed effector cells.
Figures
Figure 1
Transduction of CTLs and ATCs with GD2-specific CARs. (a) Transduction efficiency of subject-derived CTLs and ATCs by the GD2 vectors, as evaluated by quantitative PCR (qPCR, left) and FACS (right). Each symbol represents 1 of the 11 individual subjects, and the horizontal lines indicate the mean group value. Surface expression by FACS and qPCR amplification are described in Methods. (b) FACS analyses of receptor expression shown in more detail for subject 5. Percentages represent the proportion of transduced cells.
Figure 2
Immunophenotypes of CAR-transduced CTLs and ATCs. (a) Phenotypic composition of CTL and ATC population after transduction with the GD2-specific CARs. Percentages of CD4+ and CD8+ T cells, natural killer cells (CD3−CD56+) and T cells expressing TCR-γδ are shown. Each symbol represents a transduced cell line infused into a single subject. A significant difference between CTLs and ATCs was observed only for the percentage of CD8+ cells (P = 0.05). (b) Expression of naive, central memory and effector memory surface markers on GD2-specific CAR-CTLs and CAR-ATCs. The data are means ± s.d. (c) Expression of chemokine receptors and adhesion molecules on GD2-specific CAR-CTLs and CAR-ATCs. The data are means ± s.d. (d) Results of standard 51Cr release assay at an effector:tumor cell (E:T) ratio of 20:1. Data represent the mean ± s.d. percentage of specific chromium released from the CAR-CTLs and CAR-ATCs generated from each of the 11 subjects. Targets were autologous LCLs, allogeneic LCLs, autologous PHA blasts and LAN-1 cells.
Figure 3
In vivo persistence of infused CAR-CTLs versus CAR-ATCs in peripheral blood as determined by real-time quantitative PCR. A comparison of mean ± s.e.m. areas under the curve of the qPCR signal for ATCs and CTLs detected in PBMCs of treated subjects at the indicated times after infusion is shown. Five of the subjects (7 –11) received monoclonal antibodies to the common leukocyte antigen (CD45) at 2–3 d before ATC and CTL infusion and had 44–91% depletion of endogenous circulating lymphocytes. There were no measurable differences between the areas under the curve for either ATCs or CTLs between subjects 1–6 (no CD45) and 7–11 (CD45-treated).
Figure 4
Reactivation of CAR-CTLs ex vivo. (a,b) Fold change in the level of GD2-receptor-transgene positivity in the cultures before and after exposure to EBV antigen in ATCs (a) and CTLs (b). PBMCs were collected at increasing times after infusion (indicated on the x axis) and re-expanded ex vivo in the presence of EBV+ targets (total of three or four stimulations). Each symbol represents a single subject. (c) Change in percentage of cells expressing the GD2 chimeric receptor on _ex vivo-_reactivated CTLs. The y axis for the top panels indicates the isotype control, whereas for the bottom panels it indicates antibody 1A7. Percentages represent the proportions of cells expressing the CAR. (d) Cytotoxicity of _ex vivo-_reactivated CTLs against autologous LCLs, allogeneic LCLs or LAN-1 cells. The data are means ± s.d. of triplicate experiments.
Figure 5
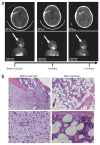
Resolution of neuroblastoma in subjects 3 and 6 after infusion of genetically engineered T cells. (a) Sequential anatomic (MRI) and functional (MIBG) imaging of the head and neck of subject 3, a 4-year-old girl with relapsed metastatic neuroblastoma, with an extradural mass and overlying calvarial bone involvement. Progressive resolution of the extradural mass and loss of MIBG uptake in the lesion by 4 months after infusion are indicated by arrows. The signal in the salivary glands remains due to normal uptake. (b) H&E stain showing postinfusion normalization of bone marrow in subject 6 after extensive infiltration by neuroblasts.
Comment in
- Epstein-Barr virus sustains tumor killers.
O'Reilly RJ. O'Reilly RJ. Nat Med. 2008 Nov;14(11):1148-50. doi: 10.1038/nm1108-1148. Nat Med. 2008. PMID: 18989276 No abstract available.
Similar articles
- Target antigen expression on a professional antigen-presenting cell induces superior proliferative antitumor T-cell responses via chimeric T-cell receptors.
Rossig C, Bär A, Pscherer S, Altvater B, Pule M, Rooney CM, Brenner MK, Jürgens H, Vormoor J. Rossig C, et al. J Immunother. 2006 Jan-Feb;29(1):21-31. doi: 10.1097/01.cji.0000175492.28723.d6. J Immunother. 2006. PMID: 16365597 - Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease.
Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, Zhang L, Heslop HE, Brenner MK, Dotti G. Savoldo B, et al. Blood. 2007 Oct 1;110(7):2620-30. doi: 10.1182/blood-2006-11-059139. Epub 2007 May 16. Blood. 2007. PMID: 17507664 Free PMC article. - Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma.
Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu MF, Gee AP, Mei Z, Rooney CM, Heslop HE, Brenner MK. Louis CU, et al. Blood. 2011 Dec 1;118(23):6050-6. doi: 10.1182/blood-2011-05-354449. Epub 2011 Oct 7. Blood. 2011. PMID: 21984804 Free PMC article. Clinical Trial. - Targeting of tumor cells by lymphocytes engineered to express chimeric receptor genes.
Baxevanis CN, Papamichail M. Baxevanis CN, et al. Cancer Immunol Immunother. 2004 Oct;53(10):893-903. doi: 10.1007/s00262-004-0523-y. Epub 2004 May 26. Cancer Immunol Immunother. 2004. PMID: 15168086 Free PMC article. Review. - Immunotherapy for Epstein-Barr virus-associated cancers in children.
Straathof KC, Bollard CM, Rooney CM, Heslop HE. Straathof KC, et al. Oncologist. 2003;8(1):83-98. doi: 10.1634/theoncologist.8-1-83. Oncologist. 2003. PMID: 12604735 Review.
Cited by
- Immunotherapy for malignant glioma.
Suryadevara CM, Verla T, Sanchez-Perez L, Reap EA, Choi BD, Fecci PE, Sampson JH. Suryadevara CM, et al. Surg Neurol Int. 2015 Feb 13;6(Suppl 1):S68-77. doi: 10.4103/2152-7806.151341. eCollection 2015. Surg Neurol Int. 2015. PMID: 25722935 Free PMC article. - Chimeric antigen receptor--modified T cells: clinical translation in stem cell transplantation and beyond.
Riddell SR, Jensen MC, June CH. Riddell SR, et al. Biol Blood Marrow Transplant. 2013 Jan;19(1 Suppl):S2-5. doi: 10.1016/j.bbmt.2012.10.021. Epub 2012 Oct 17. Biol Blood Marrow Transplant. 2013. PMID: 23085599 Free PMC article. Review. No abstract available. - Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning.
Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. Pegram HJ, et al. Blood. 2012 May 3;119(18):4133-41. doi: 10.1182/blood-2011-12-400044. Epub 2012 Feb 21. Blood. 2012. PMID: 22354001 Free PMC article. - Muscle CARs and TcRs: turbo-charged technologies for the (T cell) masses.
Kalos M. Kalos M. Cancer Immunol Immunother. 2012 Jan;61(1):127-35. doi: 10.1007/s00262-011-1173-5. Epub 2011 Dec 1. Cancer Immunol Immunother. 2012. PMID: 22131062 Free PMC article. Review. - Engineered T cells: the promise and challenges of cancer immunotherapy.
Fesnak AD, June CH, Levine BL. Fesnak AD, et al. Nat Rev Cancer. 2016 Aug 23;16(9):566-81. doi: 10.1038/nrc.2016.97. Nat Rev Cancer. 2016. PMID: 27550819 Free PMC article. Review.
References
- Heslop HE, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. - PubMed
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. - PubMed
- Khanna R, Moss D, Gandhi M. Technology insight: applications of emerging immunotherapeutic strategies for Epstein-Barr virus–associated malignancies. Nat Clin Pract Oncol. 2005;2:138–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 RR000188/RR/NCRR NIH HHS/United States
- RR00188/RR/NCRR NIH HHS/United States
- P01 CA094237/CA/NCI NIH HHS/United States
- M01 RR000188/RR/NCRR NIH HHS/United States
- P01CA94237/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical