Striatum and pre-SMA facilitate decision-making under time pressure - PubMed (original) (raw)
Comparative Study
. 2008 Nov 11;105(45):17538-42.
doi: 10.1073/pnas.0805903105. Epub 2008 Nov 3.
Affiliations
- PMID: 18981414
- PMCID: PMC2582260
- DOI: 10.1073/pnas.0805903105
Comparative Study
Striatum and pre-SMA facilitate decision-making under time pressure
Birte U Forstmann et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Human decision-making almost always takes place under time pressure. When people are engaged in activities such as shopping, driving, or playing chess, they have to continually balance the demands for fast decisions against the demands for accurate decisions. In the cognitive sciences, this balance is thought to be modulated by a response threshold, the neural substrate of which is currently subject to speculation. In a speed decision-making experiment, we presented participants with cues that indicated different requirements for response speed. Application of a mathematical model for the behavioral data confirmed that cueing for speed lowered the response threshold. Functional neuroimaging showed that cueing for speed activates the striatum and the pre-supplementary motor area (pre-SMA), brain structures that are part of a closed-loop motor circuit involved in the preparation of voluntary action plans. Moreover, activation in the striatum is known to release the motor system from global inhibition, thereby facilitating faster but possibly premature actions. Finally, the data show that individual variation in the activation of striatum and pre-SMA is selectively associated with individual variation in the amplitude of the adjustments in the response threshold estimated by the mathematical model. These results demonstrate that when people have to make decisions under time pressure their striatum and pre-SMA show increased levels of activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Paradigm outline. Moving dots paradigm with cues emphasizing speed (SN for schnell), both speed and accuracy, that is, neutral (NE) and accuracy (AK for akkurat).
Fig. 2.
Behavioral data, LBA model, and LBA model fits. (A) Behavioral results. Cueing for speed leads to decrease in response time and an increase in errors. (B) In the LBA model, the decision to respond either left or right is modeled as a race between 2 accumulators. Activation in each accumulator begins at a random point between zero and A and increases with time. The rate of increase is random from trial to trial, but is (on average) faster for the accumulator whose associated response matches the stimulus. A response is given by whichever accumulator first reaches the threshold b, and the predicted response time depends on the time taken to reach that threshold. (C) Model fit. Quantiles estimated from data (circles) and predicted by the LBA model (crosses with lines). Data are shown from 3 different response caution conditions. The upper lines and symbols show quantile estimates for correct responses, and the lower set are for incorrect responses. The data and model predictions were averaged across participants and across left vs. right stimuli.
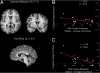
Fig. 3.
Conjunction and correlation analyses. Activation maps averaged over 19 participants mapped onto an individual brain. Red labels indicate positive Z values. Coordinates are given in Talairach space. (A) Activation elicited in the conjunction analysis of both Speed vs. Accuracy and Speed vs. Neutral. (B) Association between the individual percent signal changes derived from the right anterior striatum and the individual changes in the LBA measure for response caution. (C) Association between the individual percent signal changes derived from the right pre-SMA and the individual changes in the LBA measure for response caution.
Similar articles
- The modulatory role of pre-SMA in speed-accuracy tradeoff: A bi-directional TMS study.
Berkay D, Eser HY, Sack AT, Çakmak YÖ, Balcı F. Berkay D, et al. Neuropsychologia. 2018 Jan 31;109:255-261. doi: 10.1016/j.neuropsychologia.2017.12.031. Epub 2017 Dec 20. Neuropsychologia. 2018. PMID: 29274342 - Action sets and decisions in the medial frontal cortex.
Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Rushworth MF, et al. Trends Cogn Sci. 2004 Sep;8(9):410-7. doi: 10.1016/j.tics.2004.07.009. Trends Cogn Sci. 2004. PMID: 15350242 Review. - Adjustments of response threshold during task switching: a model-based functional magnetic resonance imaging study.
Mansfield EL, Karayanidis F, Jamadar S, Heathcote A, Forstmann BU. Mansfield EL, et al. J Neurosci. 2011 Oct 12;31(41):14688-92. doi: 10.1523/JNEUROSCI.2390-11.2011. J Neurosci. 2011. PMID: 21994385 Free PMC article. - Striatal activation reflects urgency in perceptual decision making.
van Maanen L, Fontanesi L, Hawkins GE, Forstmann BU. van Maanen L, et al. Neuroimage. 2016 Oct 1;139:294-303. doi: 10.1016/j.neuroimage.2016.06.045. Epub 2016 Jun 26. Neuroimage. 2016. PMID: 27355435 - Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal.
Frank MJ, Claus ED. Frank MJ, et al. Psychol Rev. 2006 Apr;113(2):300-326. doi: 10.1037/0033-295X.113.2.300. Psychol Rev. 2006. PMID: 16637763 Review.
Cited by
- The speed-accuracy tradeoff in the elderly brain: a structural model-based approach.
Forstmann BU, Tittgemeyer M, Wagenmakers EJ, Derrfuss J, Imperati D, Brown S. Forstmann BU, et al. J Neurosci. 2011 Nov 23;31(47):17242-9. doi: 10.1523/JNEUROSCI.0309-11.2011. J Neurosci. 2011. PMID: 22114290 Free PMC article. - Changes in neural connectivity underlie decision threshold modulation for reward maximization.
Green N, Biele GP, Heekeren HR. Green N, et al. J Neurosci. 2012 Oct 24;32(43):14942-50. doi: 10.1523/JNEUROSCI.0573-12.2012. J Neurosci. 2012. PMID: 23100417 Free PMC article. Clinical Trial. - A generative joint model for spike trains and saccades during perceptual decision-making.
Cassey PJ, Gaut G, Steyvers M, Brown SD. Cassey PJ, et al. Psychon Bull Rev. 2016 Dec;23(6):1757-1778. doi: 10.3758/s13423-016-1056-z. Psychon Bull Rev. 2016. PMID: 27246091 - Bias in the brain: a diffusion model analysis of prior probability and potential payoff.
Mulder MJ, Wagenmakers EJ, Ratcliff R, Boekel W, Forstmann BU. Mulder MJ, et al. J Neurosci. 2012 Feb 15;32(7):2335-43. doi: 10.1523/JNEUROSCI.4156-11.2012. J Neurosci. 2012. PMID: 22396408 Free PMC article. Clinical Trial. - The expected value of control: an integrative theory of anterior cingulate cortex function.
Shenhav A, Botvinick MM, Cohen JD. Shenhav A, et al. Neuron. 2013 Jul 24;79(2):217-40. doi: 10.1016/j.neuron.2013.07.007. Neuron. 2013. PMID: 23889930 Free PMC article. Review.
References
- Wickelgren WA. Speed-accuracy tradeoff and information processing dynamics. Acta Psychol. 1977;41:67–85.
- Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. The physics of optimal decision making: A formal analysis of models of performance in two-alternative forced choice tasks. Psychol Rev. 2006;113:700–765. - PubMed
- Ratcliff R. A theory of memory retrieval. Psychol Rev. 1978;85:59–108.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources