Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling - PubMed (original) (raw)
Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling
Yan Li et al. Genes Dev. 2008.
Abstract
Cell identity and tissue morphogenesis are tightly orchestrated during organogenesis, but the mechanisms regulating this are poorly understood. We show that interactions between Wnt11 and the secreted Wnt antagonist secreted frizzled-related protein 5 (Sfrp5) coordinate cell fate and morphogenesis during Xenopus foregut development. sfrp5 is expressed in the surface cells of the foregut epithelium, whereas wnt11 is expressed in the underlying deep endoderm. Depletion of Sfrp5 results in reduced foregut gene expression and hypoplastic liver and ventral pancreatic buds. In addition, the ventral foregut cells lose adhesion and fail to form a polarized epithelium. We show that the cell fate and epithelial defects are due to inappropriate Wnt/beta-catenin and Wnt/PCP signaling, respectively, both mediated by Wnt11. We provide evidence that Sfrp5 locally inhibits Wnt11 to maintain early foregut identity and to allow an epithelium to form over a mass of tissue undergoing Wnt-mediated cell movements. This novel mechanism coordinating canonical and noncanonical Wnt signaling may have broad implications for organogenesis and cancer.
Figures
Figure 1.
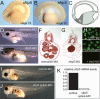
Sfrp5 is required for Xenopus gut development. (A,B) Sfrp5 in situ hybridization to stage 15 (neurula) (A) and stage 22 (∼10 somites) bisected embryos (B) shows expression in the archenteron floor of the ventral foregut. (C) Schematic of a bisected embryo indicating the endoderm (gray) and the ventral foregut (fg). Anterior is left and dorsal is up. (D) Normal intestinal coiling and a translucent hindgut in 3-d-old laevis embryos that were injected with a control mismatch-MO (50 ng). (E) Three-day-old laevis embryos that were injected with a translation-inhibiting sfrp5-MO (50 ng) exhibit a lack of gut coiling and an expanded hindgut (∼80%, n > 300). (F,G) H&E-stained transverse section of a mismatch-MO embryo (F) and a sfrp5-MO-injected embryo (G) that lacks gut coiling and has no lumen. (H) GFP fluorescence in stage 15 whole embryos injected with a synthetic sfrp5:gfp RNA containing the sfrp5 5′-UTR fused to a GFP coding sequence. Coinjection of the mismatch-MO did not inhibit GFP translation, while the sfrp5-MO blocked fluorescence. (I,J) Xenopus tropicalis embryos injected with a control-MO (I) or a sfrp5 splice-MO (J), which causes gut defects similar to the translation blocking sfrp5-MO in laevis (82%, n = 50). (K) A representative QRT–PCR experiment with primers spanning the Xt. Sfrp5 exon1–exon2 junction shows that the sfrp5 splice-MO reduces mature sfrp5 transcripts by >90% in tropicalis embryos. Five embryos were pooled for each sample, and expression was normalized to odc loading control.
Figure 2.
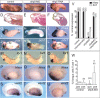
Sfrp5 is required for foregut organogenesis. (A,D,G,J,M,P,S) Control embryos. (B,E,H,K,N,Q,T) Foregut development was impaired by injection of the sfrp5-MO (50 ng). (C,F,I,L,O,R,U) Foregut development was expanded by injection of ectopic sfrp5 RNA (500 pg) into the hindgut. Anterior is to the left and dorsal is up. (A–C) External morphology of stage 30 tailbud embryos. (D–F) Mid-sagittal H&E-stained sections of stage 30 embryos showing the collapsed foregut cavity in sfrp5-MO embryos (shown in E). (G–L) In situ hybridization to isolated gut tubes at stage 42 showing that the _hhex_-expressing liver bud (G–I) and _pdx1_-expressing ventral pancreatic bud (J–L) were hypoplastic in Sfrp5-depleted embryos and expanded by Sfrp5 overexpression. (M–O) The liver specification marker for1 was repressed by sfrp5 depletion and expanded by sfrp5 overexpression at stage 35. (P–R) At stage 20, the foregut marker hhex was repressed by sfrp5 depletion and expanded by sfrp5 overexpression. (S–U) At stage 20, the Wnt/β-catenin posterior target gene vent2 was ectopically expressed in the foregut of sfrp5-MO embryos (arrow) and repressed by sfrp5 overexpression. (V) Injection of the mismatch-MO (50 ng) had no effect, while coinjection of a synthetic sfrp5 RNA lacking the sequence targeted by the sfrp5-MO rescued hhex and for1 expression in sfrp5-depleted embryos. MOs were injected at the eight-cell stage followed by a targeted injection of the sfrp5 RNA (lacking the 5′-UTR; 100 pg) into the anterior endoderm D1 cells of the 32-cell stage. The histogram shows the percent of embryos with normal expression levels. The number of embryos for each condition is in parentheses, and representative examples are shown in Supplemental Figure S2. (W) Sfrp5-depleted embryos show increased cell death in the foregut at later stages of development, but not at stage 20. The percentage of TUNEL-positive foregut cells is presented ±standard deviation. The number of embryos counted is in parentheses, with ∼75 foregut cells per embryo at stage 20 and ∼150 foregut cells per embryo at stage 35. (*) P < 0.05 compared with age-matched controls in a Student _t_-test.
Figure 3.
Ectopic Sfrp5 represses hindgut morphogenesis. (A) Hindgut elongation assay. Ventral foregut and hindgut explants were isolated at stage 15 from control embryos and embryos injected with sfrp5, dkk1, or dsh-ΔPDZ RNA and were cultured until stage 26. The length of each explant was measured before and after culturing, and the elongation was calculated as a ratio of the lengths at stage 26/stage 15. (B–E) Representative hindgut explants injected with the indicated RNAs. Bars, 0.5 mm. (F) Sfrp5 and dsh-ΔPDZ blocked hindgut elongation, while dkk1 had no effect. The histogram shows the average elongation ± standard deviation. In pairwise Student _t_-tests, (*) P < 0.001, control foregut compared with control hindgut; (**) P < 0.005 compared with control hindgut.
Figure 4.
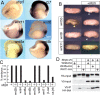
Sfrp5 interacts with Wnt5 and Wnt11. (A) In situ hybridization with the indicated probes to bisected neurula embryos showed that wnt11 and fz7 are expressed in the foregut endoderm (red arrow) underlying the sfrp5 expressing cells. (lpm) Lateral plate mesoderm. (B) Coinjection of wnt5b or wnt11 plasmids into the endoderm rescued the enlarged foregut (white arrow) caused by sfrp5 RNA overexpression, while wnt8 did not. Enlarged hindgut (yellow arrow). (C) Summary of the rescue experiments. Embryos were injected with or without sfrp5 RNA and the indicated wnt plasmid. The histogram shows the percentage of embryos with expanded foreguts. Wnt5a, Wnt5b, Wnt11, and a constitutively active JNK, rescued the Sfrp5 overexpression phenotype, whereas Wnt2b, Wnt4, Wnt7b, Wnt8 and a stabilized β-catenin did not. n > 15 embryos for in each condition. (D) Western blotting of a coimmunoprecipitation showing that V5-tagged Sfrp5 preferentially bound to HA-tagged Wnt5a and Wnt11 from transfected COS-1 cell extracts.
Figure 5.
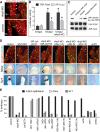
Sfrp5 is required to establish a foregut epithelium. (A,B) Removal of the neural plate at stage 13 reveals the archenteron floor foregut endoderm in control embryos (A) is a flat epithelial sheet of cells, while in sfrp5-MO embryos (B) the cells round up and are loosely adherent. Embryos are oriented anterior down. (C–H) Confocal immunostaining of anti-β-catenin (C,F), anti-atypical-PKC (D,G), and anti-C-cadherin (E,H) in the foregut region of midsagittally bisected control (C–E) and sfrp5-MO-injected (F–H) embryos at stage 20 (anterior left, dorsal up). Nuclei are counterstained in green. Insets show a high magnification of the foregut surface cells. (I–T) Confocal immunostaining of aPKC (I–N) and β-catenin (O–T) in the foregut and hindgut surface of control and Sfrp5-depleted embryos (anterior left) at the indicated stages. (U–W) The schematic shows the regions of the foregut (blue box) and hindgut (yellow box) imaged in I–T. The red line indicates the regions of the foregut and hindgut surface that exhibit a polarized epithelium with basolateral β-catenin and apical aPKC. The loss of Sfrp5 results in a failure of the foregut epithelium, reduced cell adhesion and a loss of apical–basal polarity. (fgc) Foregut cavity; (ar) archenteron; (bc) blastocoel.
Figure 6.
Sfrp5 coordinates foregut morphogenesis and specification by restricting noncanonical Wnt/JNK and canonical Wnt/β-catenin, respectively. (A) Membrane-localized Dsh is elevated in Sfrp5-depleted foregut cells. RNA encoding myc-tagged Dsh was injected into the anterior endoderm of control or sfrp5-MO-injected embryos, and its subcellular localization was determined by anti-myc immunostaining at stage 18. In control embryos, membrane-localized Dsh-myc was observed in the deep endoderm cells (yellow arrow) but not in the surface epithelium next to the foregut cavity (fgc). Foci of membrane-localized Dsh-myc were detected in the dissociating surface cells of Sfrp5-depleted foreguts (white arrows). (B) Depletion of sfrp5 results in a specific increase in β-catenin/Tcf and JNK/AP1 activity in the foregut. TOP: flash or AP1:Luciferase reporter plasmids were injected into either the D1 foregut endoderm cells or the D4 hindgut endoderm cells at the 32-cell stage of control or Sfrp5-depleted embryos. The TOP:Flash reporter is an indicator of β-catenin/Tcf activity, while the AP1:luciferase reporter is an indicator of JNK activation of c-Jun, a component of the AP1 complex. At stage 20, the reporter activity was determined by luciferase assays, in triplicate. The average values normalized to coinjected pRTK:Renila + standard deviation. (*) P < 0.05 in Student _t_-test compared with control foreguts. (C) JNK activity is elevated in Sfrp5-depleted foreguts. Foregut explants were isolated from controls, sfrp5-MO-injected embryos, or embryos treated with a JNK inhibitor (SP600125). The Western blot shows the results of a phospho-c-jun JNK activity assay. (D) Sfrp5 inhibits Wnt/β-catenin signaling to maintain foregut gene expression, and inhibits Wnt/PCP signaling to maintain foregut epithelial integrity. The indicated constructs were injected into the D1 foregut endoderm cells at the 32-cell stage. At stage 18, bisected embryos were assayed by anti-β-catenin immunostaining or by hhex and for1 in situ at stages 18 and 35, respectively. (E) Representative examples are shown, and a summary is presented in the graph below. A complete summary of all the rescue experiments and controls is presented in Supplemental Table S1.
Figure 7.
Sfrp5 antagonizes endogenous Wnt11 signaling. (A) Reducing endogenous Wnt11 rescues Sfrp5-depleted embryos. Embryos were injected in the D1 anterior endoderm at the 32-cell stage with a wnt11-MO, sfrp5-MO, or both the wnt11-MO and the sfrp5-MO. At stage 18, the foregut epithelium was assayed by anti−β-catenin or aPKC immunostaining of bisected embryos and gene expression was examined by in situ hybridization. Injection of the wnt11-MO expanded hhex and reduced vent2 expression. Coinjection of the wnt11-MO and sfrp5-MO rescued both the epithelial integrity and the changes in gene expression observed with sfrp5-MO alone. (fgc) Foregut cavity. (B) A model of how Sfrp5 restricts both Wnt11/β-catenin and Wnt11/PCP activity to coordinate foregut specification and morphogenesis.
Similar articles
- Ryk cooperates with Frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements.
Kim GH, Her JH, Han JK. Kim GH, et al. J Cell Biol. 2008 Sep 22;182(6):1073-82. doi: 10.1083/jcb.200710188. J Cell Biol. 2008. PMID: 18809723 Free PMC article. - Different thresholds of Wnt-Frizzled 7 signaling coordinate proliferation, morphogenesis and fate of endoderm progenitor cells.
Zhang Z, Rankin SA, Zorn AM. Zhang Z, et al. Dev Biol. 2013 Jun 1;378(1):1-12. doi: 10.1016/j.ydbio.2013.02.024. Epub 2013 Apr 3. Dev Biol. 2013. PMID: 23562607 Free PMC article. - The CapZ interacting protein Rcsd1 is required for cardiogenesis downstream of Wnt11a in Xenopus laevis.
Hempel A, Kühl SJ, Rothe M, Rao Tata P, Sirbu IO, Vainio SJ, Kühl M. Hempel A, et al. Dev Biol. 2017 Apr 1;424(1):28-39. doi: 10.1016/j.ydbio.2017.02.014. Epub 2017 Feb 22. Dev Biol. 2017. PMID: 28237811 - Noncanonical Wnt11 signaling and cardiomyogenic differentiation.
Flaherty MP, Dawn B. Flaherty MP, et al. Trends Cardiovasc Med. 2008 Oct;18(7):260-8. doi: 10.1016/j.tcm.2008.12.001. Trends Cardiovasc Med. 2008. PMID: 19232955 Free PMC article. Review. - Mesoderm and endoderm internalization in the Xenopus gastrula.
Winklbauer R. Winklbauer R. Curr Top Dev Biol. 2020;136:243-270. doi: 10.1016/bs.ctdb.2019.09.002. Epub 2019 Nov 5. Curr Top Dev Biol. 2020. PMID: 31959290 Review.
Cited by
- Wnt11b is involved in cilia-mediated symmetry breakage during Xenopus left-right development.
Walentek P, Schneider I, Schweickert A, Blum M. Walentek P, et al. PLoS One. 2013 Sep 13;8(9):e73646. doi: 10.1371/journal.pone.0073646. eCollection 2013. PLoS One. 2013. PMID: 24058481 Free PMC article. - The Wnt antagonist and secreted frizzled-related protein 5: implications on lipid metabolism, inflammation, and type 2 diabetes mellitus.
Liu LB, Chen XD, Zhou XY, Zhu Q. Liu LB, et al. Biosci Rep. 2018 Jul 2;38(4):BSR20180011. doi: 10.1042/BSR20180011. Print 2018 Aug 31. Biosci Rep. 2018. PMID: 29789397 Free PMC article. Review. - Frogs as integrative models for understanding digestive organ development and evolution.
Womble M, Pickett M, Nascone-Yoder N. Womble M, et al. Semin Cell Dev Biol. 2016 Mar;51:92-105. doi: 10.1016/j.semcdb.2016.02.001. Epub 2016 Feb 3. Semin Cell Dev Biol. 2016. PMID: 26851628 Free PMC article. Review. - Whole-Genome Scanning for Selection Signatures Reveals Candidate Genes Associated with Growth and Tail Length in Sheep.
Li T, Jin M, Wang H, Zhang W, Yuan Z, Wei C. Li T, et al. Animals (Basel). 2024 Feb 22;14(5):687. doi: 10.3390/ani14050687. Animals (Basel). 2024. PMID: 38473071 Free PMC article. - Genomic integration of Wnt/β-catenin and BMP/Smad1 signaling coordinates foregut and hindgut transcriptional programs.
Stevens ML, Chaturvedi P, Rankin SA, Macdonald M, Jagannathan S, Yukawa M, Barski A, Zorn AM. Stevens ML, et al. Development. 2017 Apr 1;144(7):1283-1295. doi: 10.1242/dev.145789. Epub 2017 Feb 20. Development. 2017. PMID: 28219948 Free PMC article.
References
- Bilic J., Huang Y.L., Davidson G., Zimmermann T., Cruciat C.M., Bienz M., Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. - PubMed
- Bort R., Signore M., Tremblay K., Barbera J.P., Zaret K.S. Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev. Biol. 2006;290:44–56. - PubMed
- Boutros M., Paricio N., Strutt D.I., Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. - PubMed
- Caneparo L., Huang Y.L., Staudt N., Tada M., Ahrendt R., Kazanskaya O., Niehrs C., Houart C. Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/β catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes & Dev. 2007;21:465–480. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases