Site-specific GlcNAcylation of human erythrocyte proteins: potential biomarker(s) for diabetes - PubMed (original) (raw)
Site-specific GlcNAcylation of human erythrocyte proteins: potential biomarker(s) for diabetes
Zihao Wang et al. Diabetes. 2009 Feb.
Abstract
Objective: O-linked N-acetylglucosamine (O-GlcNAc) is upregulated in diabetic tissues and plays a role in insulin resistance and glucose toxicity. Here, we investigated the extent of GlcNAcylation on human erythrocyte proteins and compared site-specific GlcNAcylation on erythrocyte proteins from diabetic and normal individuals.
Research design and methods: GlcNAcylated erythrocyte proteins or GlcNAcylated peptides were tagged and selectively enriched by a chemoenzymatic approach and identified by mass spectrometry. The enrichment approach was combined with solid-phase chemical derivatization and isotopic labeling to detect O-GlcNAc modification sites and to compare site-specific O-GlcNAc occupancy levels between normal and diabetic erythrocyte proteins.
Results: The enzymes that catalyze the cycling (addition and removal) of O-GlcNAc were detected in human erythrocytes. Twenty-five GlcNAcylated erythrocyte proteins were identified. Protein expression levels were compared between diabetic and normal erythrocytes. Thirty-five O-GlcNAc sites were reproducibly identified, and their site-specific O-GlcNAc occupancy ratios were calculated.
Conclusions: GlcNAcylation is differentially regulated at individual sites on erythrocyte proteins in response to glycemic status. These data suggest not only that site-specific O-GlcNAc levels reflect the glycemic status of an individual but also that O-GlcNAc site occupancy on erythrocyte proteins may be eventually useful as a diagnostic tool for the early detection of diabetes.
Figures
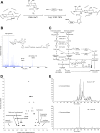
FIG. 1.
Erythrocytic proteins are O-GlcNAc modified. Fifteen micrograms of erythrocyte proteins (hemoglobin depleted) were run on a 12.5% SDS-PAGE gel, transferred, and immunoblotted with antibodies against O-GlcNAc (A), O-GlcNAcase (B, top), or OGT (B, bottom). Different lanes represent samples from different individuals.
FIG. 2.
Enrichment and identification of O-GlcNAc–modified erythrocytic proteins. A: Scheme for enriching O-GlcNAc proteins. B: Negative control of the approach. C: Confirmation of the O-GlcNAc states on several proteins.
FIG. 3.
Mapping O-GlcNAc sites and site-specific quantitation. A: Scheme for enrichment of O-GlcNAc peptides. B: Structure (inset) and CAD fragmentation of fully tagged O-GlcNAc peptide (YSPgTSPSK). [M+GlcNAc+GalNAz+Biotin+3H]3+ = 614.6, [M+H]+ = 866.5, [M+GlcNAc+H]+ = 1069.6. C: Flow chart for comparing site-specific O-GlcNAc RORs. Inset: Scheme for solid-phase BEMAD. D: Protein expression level dynamics in diabetic erythrocytes compared with normal erythrocytes. E: Specificity control for the enrichment and site-mapping. Samples were untreated or treated with hexosaminidase at 37°C for 48 h before going through the work flow. Base peak chromatograms are shown. NL, intensity in counts normalized to 1 s; TBTA, Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine; TCEP, Tris(2-carboxyethyl)phosphine.
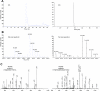
FIG. 4.
O-GlcNAc as potential biomarkers for diabetes. Specific O-GlcNAc sites (underlined Ser) on ankyrin-1 (identified and quantified by QSTAR) and catalase (identified by LTQ-Orbitrap) were upregulated 2.7- and 3.9-fold, respectively. A: Extracted ion chromatogram (XIC). B: Averaged full-scan spectra during elution time of the ion pairs. C: MS/MS spectra that showed the peptide sequences and mapped DTT attachment sites.
Similar articles
- Increased expression of beta-N-acetylglucosaminidase in erythrocytes from individuals with pre-diabetes and diabetes.
Park K, Saudek CD, Hart GW. Park K, et al. Diabetes. 2010 Jul;59(7):1845-50. doi: 10.2337/db09-1086. Epub 2010 Apr 22. Diabetes. 2010. PMID: 20413512 Free PMC article. - Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity.
Copeland RJ, Bullen JW, Hart GW. Copeland RJ, et al. Am J Physiol Endocrinol Metab. 2008 Jul;295(1):E17-28. doi: 10.1152/ajpendo.90281.2008. Epub 2008 Apr 29. Am J Physiol Endocrinol Metab. 2008. PMID: 18445751 Free PMC article. Review. - Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats.
Akimoto Y, Hart GW, Wells L, Vosseller K, Yamamoto K, Munetomo E, Ohara-Imaizumi M, Nishiwaki C, Nagamatsu S, Hirano H, Kawakami H. Akimoto Y, et al. Glycobiology. 2007 Feb;17(2):127-40. doi: 10.1093/glycob/cwl067. Epub 2006 Nov 9. Glycobiology. 2007. PMID: 17095531 - O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress.
Butkinaree C, Park K, Hart GW. Butkinaree C, et al. Biochim Biophys Acta. 2010 Feb;1800(2):96-106. doi: 10.1016/j.bbagen.2009.07.018. Epub 2009 Aug 6. Biochim Biophys Acta. 2010. PMID: 19647786 Free PMC article. Review. - O-GlcNAcylation site mapping by (azide-alkyne) click chemistry and mass spectrometry following intensive fractionation of skeletal muscle cells proteins.
Deracinois B, Camoin L, Lambert M, Boyer JB, Dupont E, Bastide B, Cieniewski-Bernard C. Deracinois B, et al. J Proteomics. 2018 Aug 30;186:83-97. doi: 10.1016/j.jprot.2018.07.005. Epub 2018 Jul 26. J Proteomics. 2018. PMID: 30016717
Cited by
- Neurons and Glia Interplay in α-Synucleinopathies.
Mavroeidi P, Xilouri M. Mavroeidi P, et al. Int J Mol Sci. 2021 May 8;22(9):4994. doi: 10.3390/ijms22094994. Int J Mol Sci. 2021. PMID: 34066733 Free PMC article. Review. - In Situ Imaging of O-Linked β-N-Acetylglucosamine Using On-Tissue Hydrolysis and MALDI Mass Spectrometry.
Escobar EE, Seeley EH, Serrano-Negrón JE, Vocadlo DJ, Brodbelt JS. Escobar EE, et al. Cancers (Basel). 2023 Feb 15;15(4):1224. doi: 10.3390/cancers15041224. Cancers (Basel). 2023. PMID: 36831567 Free PMC article. - Protein O-linked β-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function.
Darley-Usmar VM, Ball LE, Chatham JC. Darley-Usmar VM, et al. J Mol Cell Cardiol. 2012 Mar;52(3):538-49. doi: 10.1016/j.yjmcc.2011.08.009. Epub 2011 Aug 22. J Mol Cell Cardiol. 2012. PMID: 21878340 Free PMC article. Review. - Role of _O_-Linked _N_-Acetylglucosamine Protein Modification in Cellular (Patho)Physiology.
Chatham JC, Zhang J, Wende AR. Chatham JC, et al. Physiol Rev. 2021 Apr 1;101(2):427-493. doi: 10.1152/physrev.00043.2019. Epub 2020 Jul 30. Physiol Rev. 2021. PMID: 32730113 Free PMC article. Review. - O-GlcNAcylation and neurodegeneration.
Wani WY, Chatham JC, Darley-Usmar V, McMahon LL, Zhang J. Wani WY, et al. Brain Res Bull. 2017 Jul;133:80-87. doi: 10.1016/j.brainresbull.2016.08.002. Epub 2016 Aug 4. Brain Res Bull. 2017. PMID: 27497832 Free PMC article. Review.
References
- Love DC, Hanover JA: The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE 2005: re13, 2005 - PubMed
- Hart GW, Housley MP, Slawson C: Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446: 1017–1022, 2007 - PubMed
- Wells L, Vosseller K, Hart GW: Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291: 2376–2378, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 DK071280/DK/NIDDK NIH HHS/United States
- N01-HV-28180/HV/NHLBI NIH HHS/United States
- N01HV28180/HV/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R33 DK071280/DK/NIDDK NIH HHS/United States
- DK-71280/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases