Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2008 Nov 5;300(17):2012-21.
doi: 10.1001/jama.2008.555.
Affiliations
- PMID: 18984888
- PMCID: PMC2593624
- DOI: 10.1001/jama.2008.555
Randomized Controlled Trial
Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial
Shumin M Zhang et al. JAMA. 2008.
Abstract
Context: Folate, vitamin B(6), and vitamin B(12) are thought to play an important role in cancer prevention.
Objective: To evaluate the effect of combined folic acid, vitamin B(6), and vitamin B(12) treatment on cancer risk in women at high risk for cardiovascular disease.
Design, setting, and participants: In the Women's Antioxidant and Folic Acid Cardiovascular Study, 5442 US female health professionals aged 42 years or older, with preexisting cardiovascular disease or 3 or more coronary risk factors, were randomly assigned to receive either a daily combination of folic acid, vitamin B(6), and vitamin B(12) or a matching placebo. They were treated for 7.3 years from April 1998 through July 31, 2005.
Intervention: Daily supplementation of a combination of 2.5 mg of folic acid, 50 mg of vitamin B(6), and 1 mg of vitamin B(12) (n = 2721) or placebo (n = 2721).
Main outcome measures: Confirmed newly diagnosed total invasive cancer or breast cancer.
Results: A total of 379 women developed invasive cancer (187 in the active treatment group and 192 in the placebo group). Compared with placebo, women receiving the active treatment had similar risk of developing total invasive cancer (101.1/10,000 person-years for the active treatment group vs 104.3/10,000 person-years for placebo group; hazard ratio [HR], 0.97; 95% confidence interval [CI], 0.79-1.18; P = .75), breast cancer (37.8/10,000 person-years vs 45.6/10,000 person-years, respectively; HR, 0.83; 95% CI, 0.60-1.14; P = .24), or any cancer death (24.6/10,000 person-years vs 30.1/10,000 person-years, respectively; HR, 0.82; 95% CI, 0.56-1.21; P = .32).
Conclusion: Combined folic acid, vitamin B(6), and vitamin B(12) treatment had no significant effect on overall risk of total invasive cancer or breast cancer among women during the folic acid fortification era.
Trial registration: clinicaltrials.gov Identifier: NCT00000541.
Figures
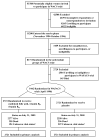
Figure 1. Flow Diagram of the Folic Acid, Vitamin B6, and Vitamin B12 Component of WAFACS Trial
Abbreviations: WACS: Women’s Antioxidant Cardiovascular Study; WAFACS: Women’s Antioxidant and Folic Acid Cardiovascular Study. a Mortality and morbidity information was complete for 98.9% and 98.0% of person-years of follow-up, respectively.
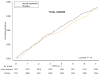
Figure 2
Cumulative Incidence Estimates of total invasive cancer (panel A) and breast cancer (panel B) (adjusted for age and randomized vitamin E, vitamin C, and beta-carotene assignments)
Figure 2
Cumulative Incidence Estimates of total invasive cancer (panel A) and breast cancer (panel B) (adjusted for age and randomized vitamin E, vitamin C, and beta-carotene assignments)
Comment in
- ACP Journal Club. Combined therapy with folic acid and vitamins B6 and B12 did not affect cancer risk in women at high risk for CV disease.
Diehl AK. Diehl AK. Ann Intern Med. 2009 Mar 17;150(6):JC3-9. doi: 10.7326/0003-4819-150-6-200903170-02009. Ann Intern Med. 2009. PMID: 19306491 No abstract available.
Similar articles
- Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial.
Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. Albert CM, et al. JAMA. 2008 May 7;299(17):2027-36. doi: 10.1001/jama.299.17.2027. JAMA. 2008. PMID: 18460663 Free PMC article. Clinical Trial. - Folic Acid, Vitamin B6, and Vitamin B12 in Combination and Age-Related Cataract in a Randomized Trial of Women.
Christen WG, Glynn RJ, Chew EY, Albert CM, Manson JE. Christen WG, et al. Ophthalmic Epidemiol. 2016;23(1):32-9. doi: 10.3109/09286586.2015.1130845. Epub 2016 Jan 20. Ophthalmic Epidemiol. 2016. PMID: 26786311 Free PMC article. Clinical Trial. - Cancer incidence and mortality after treatment with folic acid and vitamin B12.
Ebbing M, Bønaa KH, Nygård O, Arnesen E, Ueland PM, Nordrehaug JE, Rasmussen K, Njølstad I, Refsum H, Nilsen DW, Tverdal A, Meyer K, Vollset SE. Ebbing M, et al. JAMA. 2009 Nov 18;302(19):2119-26. doi: 10.1001/jama.2009.1622. JAMA. 2009. PMID: 19920236 - Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials.
Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Balk EM, et al. Arch Intern Med. 2007 Jan 8;167(1):21-30. doi: 10.1001/archinte.167.1.21. Arch Intern Med. 2007. PMID: 17210874 Review. - Vitamin supplements and cardiovascular risk: review of the randomized trials of homocysteine-lowering vitamin supplements.
Clarke R, Armitage J. Clarke R, et al. Semin Thromb Hemost. 2000;26(3):341-8. doi: 10.1055/s-2000-8101. Semin Thromb Hemost. 2000. PMID: 11011852 Review.
Cited by
- Serum Folate Correlates with Severity of Guillain-Barré Syndrome and Predicts Disease Progression.
Gao Y, Zhang HL, Xin M, Wang D, Zheng N, Wang S, Xu J, Wang Y, Zhu J, Feng J. Gao Y, et al. Biomed Res Int. 2018 Jun 14;2018:5703279. doi: 10.1155/2018/5703279. eCollection 2018. Biomed Res Int. 2018. PMID: 30013984 Free PMC article. - Genetically predicted circulating B vitamins in relation to digestive system cancers.
Yuan S, Carter P, Vithayathil M, Kar S, Mason AM, Burgess S, Larsson SC. Yuan S, et al. Br J Cancer. 2021 Jun;124(12):1997-2003. doi: 10.1038/s41416-021-01383-0. Epub 2021 Apr 9. Br J Cancer. 2021. PMID: 33837300 Free PMC article. - Effect of Combination Folic Acid, Vitamin B6 , and Vitamin B12 Supplementation on Fracture Risk in Women: A Randomized, Controlled Trial.
Stone KL, Lui LY, Christen WG, Troen AM, Bauer DC, Kado D, Schambach C, Cummings SR, Manson JE. Stone KL, et al. J Bone Miner Res. 2017 Dec;32(12):2331-2338. doi: 10.1002/jbmr.3229. J Bone Miner Res. 2017. PMID: 29244251 Free PMC article. Clinical Trial. - The folate status of reproductive-aged women in a randomised trial of a folate-fortified oral contraceptive: dietary and blood assessments.
Castaño PM, Aydemir A, Sampson-Landers C, Lynen R. Castaño PM, et al. Public Health Nutr. 2014 Jun;17(6):1375-83. doi: 10.1017/S1368980013000864. Epub 2013 Mar 27. Public Health Nutr. 2014. PMID: 23534865 Free PMC article. Clinical Trial. - Interpreting Research on Dietary Supplements and Cancer - What is the Take Home Message?
Miller PE, Andrzejewski L, Chyan W, Snyder DC. Miller PE, et al. Oncol Nutr Connect. 2009 Spring;17(3):3-9. Oncol Nutr Connect. 2009. PMID: 25242899 Free PMC article. No abstract available.
References
- Mason JB, Levesque T. Folate: effects on carcinogenesis and the potential for cancer chemoprevention. Oncology (Williston Park) 1996;10(11):1727–1736. 1742–1743. discussion 1743–1744. - PubMed
- Ames BN. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat Res. 2001;475(1–2):7–20. - PubMed
- Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340(19):1449–1454. - PubMed
- Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160(4):339–349. - PubMed
- Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002;132(8 Suppl):2350S–2355S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL046959-13S1/HL/NHLBI NIH HHS/United States
- R01 HL046959-10/HL/NHLBI NIH HHS/United States
- R01 HL046959-07/HL/NHLBI NIH HHS/United States
- R01 HL046959-13/HL/NHLBI NIH HHS/United States
- R01 HL046959/HL/NHLBI NIH HHS/United States
- R01 HL046959-09/HL/NHLBI NIH HHS/United States
- R01 HL046959-11/HL/NHLBI NIH HHS/United States
- HL47959/HL/NHLBI NIH HHS/United States
- R01 HL046959-08/HL/NHLBI NIH HHS/United States
- R01 HL046959-12/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous