Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau - PubMed (original) (raw)
Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau
Kim N Green et al. J Neurosci. 2008.
Abstract
Memory loss is the signature feature of Alzheimer's disease, and therapies that prevent or delay its onset are urgently needed. Effective preventive strategies likely offer the greatest and most widespread benefits. Histone deacetylase (HDAC) inhibitors increase histone acetylation and enhance memory and synaptic plasticity. We evaluated the efficacy of nicotinamide, a competitive inhibitor of the sirtuins or class III NAD(+)-dependent HDACs in 3xTg-AD mice, and found that it restored cognitive deficits associated with pathology. Nicotinamide selectively reduces a specific phospho-species of tau (Thr231) that is associated with microtubule depolymerization, in a manner similar to inhibition of SirT1. Nicotinamide also dramatically increased acetylated alpha-tubulin, a primary substrate of SirT2, and MAP2c, both of which are linked to increased microtubule stability. Reduced phosphoThr231-tau was related to a reduction of monoubiquitin-conjugated tau, suggesting that this posttranslationally modified form of tau may be rapidly degraded. Overexpression of a Thr231-phospho-mimic tau in vitro increased clearance and decreased accumulation of tau compared with wild-type tau. These preclinical findings suggest that oral nicotinamide may represent a safe treatment for AD and other tauopathies, and that phosphorylation of tau at Thr231 may regulate tau stability.
Figures
Figure 1.
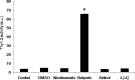
Nicotinamide does not affect thy1.2 promoter. HEK293 cells nucleofected with Thy1.2-luc and then treated with DMSO (0.1%), nicotinamide (5 m
m
), butyrate (5 m
m
), sirtinol (15 μ
m
), or Aβ42 (1 μ
m
) for 24 h. Luciferase activity was assessed and light output normalized to protein concentration and plotted. Butyrate treatment increased Thy1.2 promoter activity ∼30-fold. Error bars indicate SEM, and * indicates significance versus PBS treatment (p < 0.05).
Figure 2.
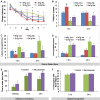
Nicotinamide prevents memory impairments in a hippocampal-dependent task in the 3xTg-AD mice. 3xTg-AD mice were treated with nicotinamide or vehicle for 4 months in their drinking water. Mice were trained and tested on the spatial memory version of the Morris water maze (MWM; n = 8 per group). A, Acquisition curves shown for the 7 d of training on the MWM. Nicotinamide prevents spatial memory deficits during training in the 3xTg-AD mice. All mice were trained to criterion in the MWM task (as indicated by dashed line at 25 s escape latency). B–D, Mice were given a memory probe with the platform removed at 1.5 h or 24 h after the last training trial. B, 3xTg-AD mice treated with nicotinamide made significantly more platform crosses at both short- and long-term probes than vehicle-treated 3xTg-AD mice. NonTg mice treated with nicotinamide performed better at the 1.5 h probe than vehicle treated NonTg mice. C, 3xTg-AD mice treated with nicotinamide exhibited significantly decreased latencies to cross the platform location compared with vehicle-treated 3xTg-AD mice, at both the 1.5 and 24 h probes. NonTg mice treated with nicotinamide had decreased latencies to cross the platform location compared with vehicle treated nontransgenic mice at only the 1.5 h probe. D, No significant differences in the time spent in the opposite quadrant were seen with nicotinamide treatment compared with vehicle for either 3xTg-AD or NonTg mice. E, Nicotinamide prevents contextual fear memory deficits in a mainly amygdala-dependent task. Mice were tested for retention of memory for fear-associated environments 1.5 and 24 h after training. Mice were taken out after 180 s if they did not cross over. F, Nicotinamide treatment does not affect cortex-dependent novel object recognition. No significant differences were seen between 3xTg-AD mice treated with nicotinamide or vehicle on their ability to remember a prior object, either 1.5 or 24 h after habituation with the object. Error bars indicate SEM. (*p < 0.05) for control 3xTg-AD mice vs nicotinamide treated 3xTg-AD mice, (**p < 0.05) for control nonTg mice vs control 3xTg-AD mice, and (#p < 0.05) for control nonTg mice vs nicotinamide treated nonTg mice.
Figure 3.
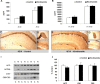
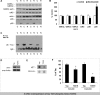
Nicotinamide treatment does not affect Aβ load or production. Soluble (A) and insoluble (B) Aβ40 and Aβ42 levels were measured from 3xTg-AD whole-brain homogenates from animals treated for 4 months with nicotinamide or vehicle. No significant differences were seen between treatments. C–H, DAB staining with 6E10 shows Aβ-like immunoreactivity in 40 μm sections from nicotinamide- and vehicle-treated mice. Staining was apparent in the hippocampal region (C, F), amygdala (D, G), and cortex (E, H; original magnification, 5×), but no differences were seen with treatment. I, Western blot analyses of protein extracts from whole-brain homogenates of 3xTg-AD mice treated for 4 months with either nicotinamide (N; n = 8) or vehicle (C; n = 8) shown as alternating lanes. Steady-state levels of APP and APP CTF's C83 and C99 were unaffected by nicotinamide treatment. J, Quantification of I normalized to β-actin levels as a loading control. Error bars indicate SEM.
Figure 4.
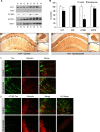
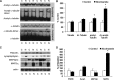
Tau pathology is decreased after nicotinamide treatment. Western blot analyses of protein extracts from whole-brain homogenates of 3xTg-AD mice treated for 4 months with either nicotinamide (N; n = 8) or vehicle (C; n = 8) shown as alternating lanes. Steady-state levels of total human tau (HT7) and tau phosphorylated at ser199/202 (AT8), thr231 (AT180), and thr181 (AT270) are shown. B, Quantification of A normalized to β-actin levels as a loading control. Error bars indicate SEM, and * indicates significance versus vehicle treatment (p < 0.05). Nicotinamide treatment dramatically reduces Thr213-phosphotau immunoreactivity. C–H, DAB staining with HT7 shows human tau immunoreactivity in 40 μm sections from nicotinamide- and vehicle-treated mice. Staining was apparent in the hippocampal region (C, F), amygdala (D, G) but not cortex (E, I; original magnification, 5×). I, J, Confocal microscopy images from 40 μm brain sections from nicotinamide- and vehicle-treated 3xTg-AD mice. CA1 pyramidal neurons shown (magnification 60×). Ubiquitin immunoreactivity shown in red for all panels. Total human tau (HT7) reactivity shown in green for I and human tau phosphorylated at thr231 (AT180) reactivity shown in green for J. Merge image shown for both total human tau and ubiquitin and for AT180-tau and ubiquitin.
Figure 5.
Nicotinamide treatment increases p25 and reduces monoubiquitinated-tau. Western blot analyses of protein extracts from whole-brain homogenates of 3xTg-AD mice treated for 4 months with either nicotinamide (N; n = 8) or vehicle (C; n = 8) shown as alternating lanes. Steady-state levels of GSK3α/β, inactive GSK3β (phosphorylated at ser9), cdk5 and p35/p25 shown. B, Quantification of A normalized to β-actin levels as a loading control. Error bars indicate SEM, and * indicates significance versus vehicle treatment (p < 0.05). C, One hundred micrograms of brain homogenate immunoprecipitated with HT7 to isolate total human tau and then probed with anti-ubiquitin. Monoubiquitinated tau is identified by the molecular weight, which is 9 kDa heavier than human tau. Mouse heavy IgG chains also shown (55 kDa). No other ubiquitin-positive bands were seen. D, Overexpression of 5 μg of either wild-type human tau or Thr231 phospho-mimic human tau (T231E) in 3T3 cells with coexpression of myc-actin as a transcriptional and loading control (n = 10 per condition). Steady-state levels of the T231E phospho-mimic are significantly reduced, whereas myc-actin levels are consistent with wild-type tau myc-actin levels showing that transcriptional production of the proteins are not affected, but rather tau stability. E, Filter retardation assay of insoluble tau shows that wild-type human tau accumulates into high molecular weight aggregates, and that this is reduced with Thr231 phosphorylation mimic T231E tau. Memcode protein staining shown as a loading control. F, Quantification of D and E. Error bars indicate SEM, and * indicates significance versus wild-type tau (p < 0.05).
Figure 6.
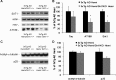
Nicotinamide treatment inhibits brain sirtuins and increase acetylated α-tubulin and MAP2c. Western blot analyses of protein extracts from whole-brain homogenates of 3xTg-AD mice treated for 4 months with either nicotinamide (N; n = 8) or vehicle (C; n = 8) shown as alternating lanes. A, Steady-state levels of acetylated α-tubulin showing an increase with nicotinamide treatment, and steady-state levels of a band corresponding to acetyl-α-tubulin dimer, which shows a large increase with nicotinamide treatment. Total steady-state levels of monomeric and dimeric α-tubulin are also shown, although no differences were evident between nicotinamide and vehicle treatment. B, Quantification of A. C, Steady-state levels of PSD-95, synaptophysin, MAP2a, MAP2b, and MAP2c shown. Increases were seen in MAP2c with nicotinamide treatment. D, Quantification of C normalized to actin as a loading control. Error bars indicate SEM, and * indicates significance versus vehicle treatment (p < 0.05).
Figure 7.
SirT1 knockdown leads to reduced Thr231-phosphotau. Whole-brain homogenates from 12-month-old 3xTg-AD hemizygous mice (n = 6) and 12-month-old 3xTg-AD mice with 1 copy of SirT1 knocked out (n = 6). A, Western blot analyses show a reduction in SirT1 protein levels and Thr231-phosphotau (AT180) in mice lacking a copy of the SirT1 gene. No changes were seen in total human tau (HT7). B, Quantification of A normalized to actin as a loading control. C, Steady-state levels of acetylated α-tubulin and p25 were not altered in mice lacking a copy of SirT1. D, Quantification of C normalized to actin as a loading control. Error bars indicate SEM, and * indicates significance versus vehicle treatment (p < 0.05).
Similar articles
- A novel glycogen synthase kinase-3 inhibitor 2-methyl-5-(3-{4-[(S )-methylsulfinyl]phenyl}-1-benzofuran-5-yl)-1,3,4-oxadiazole decreases tau phosphorylation and ameliorates cognitive deficits in a transgenic model of Alzheimer's disease.
Onishi T, Iwashita H, Uno Y, Kunitomo J, Saitoh M, Kimura E, Fujita H, Uchiyama N, Kori M, Takizawa M. Onishi T, et al. J Neurochem. 2011 Dec;119(6):1330-40. doi: 10.1111/j.1471-4159.2011.07532.x. Epub 2011 Nov 2. J Neurochem. 2011. PMID: 21992552 - Tubastatin A/ACY-1215 improves cognition in Alzheimer's disease transgenic mice.
Zhang L, Liu C, Wu J, Tao JJ, Sui XL, Yao ZG, Xu YF, Huang L, Zhu H, Sheng SL, Qin C. Zhang L, et al. J Alzheimers Dis. 2014;41(4):1193-1205. doi: 10.3233/JAD-140066. J Alzheimers Dis. 2014. PMID: 24844691 - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - [Therapeutic potential of targeting SIRT1 for the treatment of Alzheimer's disease].
Shen LL, Sun HY, Wang HQ. Shen LL, et al. Sheng Li Xue Bao. 2023 Feb 25;75(1):99-107. Sheng Li Xue Bao. 2023. PMID: 36859839 Review. Chinese.
Cited by
- Endogenous metabolism in endothelial and immune cells generates most of the tissue vitamin B3 (nicotinamide).
Zeidler JD, Chini CCS, Kanamori KS, Kashyap S, Espindola-Netto JM, Thompson K, Warner G, Cabral FS, Peclat TR, Gomez LS, Lopez SA, Wandersee MK, Schoon RA, Reid K, Menzies K, Beckedorff F, Reid JM, Brachs S, Meyer RG, Meyer-Ficca ML, Chini EN. Zeidler JD, et al. iScience. 2022 Oct 23;25(11):105431. doi: 10.1016/j.isci.2022.105431. eCollection 2022 Nov 18. iScience. 2022. PMID: 36388973 Free PMC article. - Nutrition and dementia.
Coppedè F, Bosco P, Fuso A, Troen AM. Coppedè F, et al. Curr Gerontol Geriatr Res. 2012;2012:926082. doi: 10.1155/2012/926082. Epub 2012 Jun 18. Curr Gerontol Geriatr Res. 2012. PMID: 22761616 Free PMC article. No abstract available. - SIRT1 in the brain-connections with aging-associated disorders and lifespan.
Ng F, Wijaya L, Tang BL. Ng F, et al. Front Cell Neurosci. 2015 Mar 9;9:64. doi: 10.3389/fncel.2015.00064. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25805970 Free PMC article. Review. - The plasma membrane redox system is impaired by amyloid β-peptide and in the hippocampus and cerebral cortex of 3xTgAD mice.
Hyun DH, Mughal MR, Yang H, Lee JH, Ko EJ, Hunt ND, de Cabo R, Mattson MP. Hyun DH, et al. Exp Neurol. 2010 Oct;225(2):423-9. doi: 10.1016/j.expneurol.2010.07.020. Epub 2010 Jul 27. Exp Neurol. 2010. PMID: 20673763 Free PMC article. - The effects of nicotinamide on reinstatement to cocaine seeking in male and female Sprague Dawley rats.
Witt EA, Reissner KJ. Witt EA, et al. Psychopharmacology (Berl). 2020 Mar;237(3):669-680. doi: 10.1007/s00213-019-05404-y. Epub 2019 Dec 7. Psychopharmacology (Berl). 2020. PMID: 31811351 Free PMC article.
References
- Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci U S A. 1997;94:298–303. - PMC - PubMed
- Angelo M, Plattner F, Irvine EE, Giese KP. Improved reversal learning and altered fear conditioning in transgenic mice with regionally restricted p25 expression. Eur J Neurosci. 2003;18:423–431. - PubMed
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. - PMC - PubMed
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive. 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG021982-06/AG/NIA NIH HHS/United States
- R01 NS052789/NS/NINDS NIH HHS/United States
- AG0212982/AG/NIA NIH HHS/United States
- R01 AG021982/AG/NIA NIH HHS/United States
- NS-52789/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical