Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition - PubMed (original) (raw)
Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition
Elisabeth M Zeisberg et al. J Am Soc Nephrol. 2008 Dec.
Abstract
Fibroblasts are key mediators of fibrosis in the kidney and other organs, but their origin during fibrosis is still not completely clear. Activated fibroblasts likely arise from resident quiescent fibroblasts via epithelial-to-mesenchymal transition and from the bone marrow. Here, we demonstrate that endothelial cells also contribute to the emergence of fibroblasts during kidney fibrosis via the process of endothelial-to-mesenchymal transition (EndMT). We examined the contribution of EndMT to renal fibrosis in three mouse models of chronic kidney disease: (1) Unilateral ureteral obstructive nephropathy, (2) streptozotocin-induced diabetic nephropathy, and (3) a model of Alport renal disease. Approximately 30 to 50% of fibroblasts coexpressed the endothelial marker CD31 and markers of fibroblasts and myofibroblasts such as fibroblast specific protein-1 and alpha-smooth muscle actin. Endothelial lineage tracing using Tie2-Cre;R26R-stop-EYFP transgenic mice further confirmed the presence of EndMT-derived fibroblasts. Collectively, our results demonstrate that EndMT contributes to the accumulation of activated fibroblasts and myofibroblasts in kidney fibrosis and suggest that targeting EndMT might have therapeutic potential.
Figures
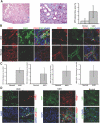
Figure 1.
EndMT in the mouse model of UUO. (A) Kidneys were analyzed after 1 wk of ureter ligation. The pictures display representative photomicrographs of MTS-stained kidney section at an original magnification of ×10 (left) and ×60 (middle). Fibrosis was digitally quantified and is shown as the percentage of MTS-stained blue area in both normal and UUO kidneys (right). (B, left) FSP1 and CD31 double labeling. Frozen kidney sections were double stained with antibodies to FSP1 (green) and CD31 (red). DAPI was used as a nuclear stain (blue). The panels display representative images that were obtained from UUO kidneys (top) and normal kidneys (bottom) using a confocal microscope. The arrows in the merged panel point to CD31+FSP1+ cells. (B, right) α-SMA and CD31 double labeling. The pictures display representative pictures of kidneys that were labeled with antibodies to α-SMA (green) and CD31 (red). DAPI was used for labeling of nuclei (blue). Yellow color in the merged panel indicates coexpression of α-SMA and CD31. The photomicrographs were obtained by confocal microscopy. (C) Quantification of fibroblasts. The bar graphs summarize average numbers of FSP1+ fibroblasts, CD31+FSP1+ cells, α-SMA+ fibroblasts, and CD31+α-SMA+ cells per visual field in both normal and obstructed kidneys at a magnification of ×63 (n = 3 mice per group, 10 high-power fields [hpf] per mouse, 30 hpf total). (D) Lineage tracing of endothelial cells. UUO was performed in Tie2-Cre;R26R-stop-EYFP double-transgenic mice. In this reporter strain, all cells of endothelial origin are tagged by YFP (shown in green). After UUO, immunostaining was performed for FSP1 (left, red) or α-SMA (middle, red). White arrows indicate fibroblasts of endothelial origin (yellow). For comparison, a representative YFP image from a normal Tie2-Cre;R26R-stop-EYFP kidney is also included (right). Magnification, ×63 in B and D.
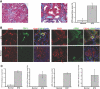
Figure 2.
EndMT in the mouse model of STZ-induced diabetic nephropathy. (A) CD1 mice were made diabetic by a single injection of STZ. Kidneys were analyzed after 6 mo. The pictures display representative photomicrographs of MTS-stained kidney sections at an original magnification of ×10 (left) and ×60 (middle). Fibrosis was digitally quantified and is shown as the percentage of MTS-stained blue area in both normal and STZ kidneys (right). (B, left) FSP1 and CD31 double labeling. Frozen kidney sections were double stained with antibodies to FSP1 (green) and CD31 (red). DAPI was used to label the nuclei (blue). The panels display representative images that were obtained from STZ kidneys (top) and normal kidneys (bottom) using a confocal microscope. The arrows in the merged panel point to CD31+FSP1+ cells. (B, right) α-SMA and CD31 double labeling. The panels display representative images of kidneys that were labeled with antibodies to α-SMA (green) and CD31 (red). Yellow color in the merged panel indicates coexpression of α-SMA and CD31. (C) Quantification of fibroblasts. The bar graphs summarize average number of FSP1+ fibroblasts, CD31+FSP1+ cells, α-SMA+ fibroblasts, and CD31+α-SMA+ cells per visual field in both normal and diabetic kidneys at a magnification of ×63 (n = 3 mice per group, 10 hpf per mouse, 30 hpf total). Magnification, ×63 in B.
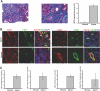
Figure 3.
EndMT in _COL4A3_-deficient mice. (A) Kidneys of COL4A3 KO mice, a mouse model for Alport syndrome, were analyzed at the age of 22 wk. The pictures display representative photomicrographs of MTS-stained kidney sections at an original magnification of ×10 (left) and ×60 (middle). Fibrosis was digitally quantified and is shown as the percentage of MTS-stained blue area in both normal and COL4A3 KO kidneys (right). (B, left) FSP1 and CD31 double labeling. Kidney sections were double stained with antibodies to FSP1 (green) and CD31 (red). DAPI was used as a nuclear stain (blue). The panels display representative images that were obtained from COL4A3 KO kidneys (top) and normal kidneys (bottom). The arrows in the merged panel point to CD31+FSP1+ cells. (B, right) α-SMA and CD31 double labeling. The pictures display representative photomicrographs of kidneys that were labeled with antibodies to α-SMA (green) and CD31 (red). DAPI was used for labeling of nuclei (blue). Yellow color in the merged panel indicates coexpression of α-SMA and CD31. (C) Quantification of fibroblasts. The bar graphs summarize average number of FSP1+ fibroblasts, CD31+FSP1+ cells, α-SMA+ fibroblasts, and CD31+α-SMA+ cells per visual field in both normal and Alport kidneys at a magnification of ×63 (n = 3 mice per group, 10 hpf per mouse, 30 hpf total). Magnification, ×63 in B.
Comment in
- How many different roads may a cell walk down in order to become a fibroblast?
Strutz F. Strutz F. J Am Soc Nephrol. 2008 Dec;19(12):2246-8. doi: 10.1681/ASN.2008101089. Epub 2008 Nov 19. J Am Soc Nephrol. 2008. PMID: 19020000 No abstract available.
Similar articles
- Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts.
Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Zeisberg EM, et al. Cancer Res. 2007 Nov 1;67(21):10123-8. doi: 10.1158/0008-5472.CAN-07-3127. Cancer Res. 2007. PMID: 17974953 - Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice.
Li J, Qu X, Bertram JF. Li J, et al. Am J Pathol. 2009 Oct;175(4):1380-8. doi: 10.2353/ajpath.2009.090096. Epub 2009 Sep 3. Am J Pathol. 2009. PMID: 19729486 Free PMC article. - Endothelial-to-mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and Akt pathway.
Curci C, Castellano G, Stasi A, Divella C, Loverre A, Gigante M, Simone S, Cariello M, Montinaro V, Lucarelli G, Ditonno P, Battaglia M, Crovace A, Staffieri F, Oortwijn B, van Amersfoort E, Gesualdo L, Grandaliano G. Curci C, et al. Nephrol Dial Transplant. 2014 Apr;29(4):799-808. doi: 10.1093/ndt/gft516. Epub 2014 Jan 23. Nephrol Dial Transplant. 2014. PMID: 24463188 - The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis.
Sun YB, Qu X, Caruana G, Li J. Sun YB, et al. Differentiation. 2016 Sep;92(3):102-107. doi: 10.1016/j.diff.2016.05.008. Epub 2016 Jun 1. Differentiation. 2016. PMID: 27262400 Review. - Epithelial to Mesenchymal Transition (EMT) and Endothelial to Mesenchymal Transition (EndMT): Role and Implications in Kidney Fibrosis.
Cruz-Solbes AS, Youker K. Cruz-Solbes AS, et al. Results Probl Cell Differ. 2017;60:345-372. doi: 10.1007/978-3-319-51436-9_13. Results Probl Cell Differ. 2017. PMID: 28409352 Review.
Cited by
- Single-cell transcriptomic profiling reveals decreased ER protein Reticulon3 drives the progression of renal fibrosis.
Guo S, Dong Y, Du R, Liu YX, Liu S, Wang Q, Liu JS, Xu H, Jiang YJ, Hao H, Fan LL, Xiang R. Guo S, et al. Mol Biomed. 2024 Jun 28;5(1):24. doi: 10.1186/s43556-024-00187-x. Mol Biomed. 2024. PMID: 38937317 Free PMC article. - Fibroblasts as architects of cancer pathogenesis.
Marsh T, Pietras K, McAllister SS. Marsh T, et al. Biochim Biophys Acta. 2013 Jul;1832(7):1070-8. doi: 10.1016/j.bbadis.2012.10.013. Epub 2012 Oct 30. Biochim Biophys Acta. 2013. PMID: 23123598 Free PMC article. Review. - Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease.
von Gise A, Pu WT. von Gise A, et al. Circ Res. 2012 Jun 8;110(12):1628-45. doi: 10.1161/CIRCRESAHA.111.259960. Circ Res. 2012. PMID: 22679138 Free PMC article. Review. - Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis.
Kanasaki K, Taduri G, Koya D. Kanasaki K, et al. Front Endocrinol (Lausanne). 2013 Feb 6;4:7. doi: 10.3389/fendo.2013.00007. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23390421 Free PMC article. - Ephrin B2 mediates high glucose induced endothelial-to-mesenchymal transition in human aortic endothelial cells.
Yuan C, Ni L, Zhang C, Xia H, Wu X. Yuan C, et al. Cardiovasc Diagn Ther. 2020 Aug;10(4):778-785. doi: 10.21037/cdt-20-299. Cardiovasc Diagn Ther. 2020. PMID: 32968633 Free PMC article.
References
- Harris RC, Neilson EG: Toward a unified theory of renal progression. Annu Rev Med 57: 365–380, 2006 - PubMed
- Zeisberg M, Kalluri R: Experimental strategies to reverse chronic renal disease. Blood Purif 22: 440–445. 2004 - PubMed
- Zeisberg M, Strutz F, Muller GA: Renal fibrosis: An update. Curr Opin Nephrol Hypertens 10: 315–320, 2001 - PubMed
- Strutz F, Zeisberg M: Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 17: 2992–2998, 2006 - PubMed
- Eddy AA: Molecular insights into renal interstitial fibrosis [Editorial]. J Am Soc Nephrol 7: 2495–2508, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK61688/DK/NIDDK NIH HHS/United States
- DK55001/DK/NIDDK NIH HHS/United States
- T32 GM007226/GM/NIGMS NIH HHS/United States
- R01 AA013913/AA/NIAAA NIH HHS/United States
- 1K08 CA129204/CA/NCI NIH HHS/United States
- R01 DK061688/DK/NIDDK NIH HHS/United States
- K08 DK074558/DK/NIDDK NIH HHS/United States
- CA12550/CA/NCI NIH HHS/United States
- R01 DK062987/DK/NIDDK NIH HHS/United States
- R01 DK055001/DK/NIDDK NIH HHS/United States
- GM07226/GM/NIGMS NIH HHS/United States
- DK62987/DK/NIDDK NIH HHS/United States
- AA13913/AA/NIAAA NIH HHS/United States
- K08 CA129204/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous