The molecular face of lipid rafts in model membranes - PubMed (original) (raw)
The molecular face of lipid rafts in model membranes
H Jelger Risselada et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Cell membranes contain a large number of different lipid species. Such a multicomponent mixture exhibits a complex phase behavior with regions of structural and compositional heterogeneity. Especially domains formed in ternary mixtures, composed of saturated and unsaturated lipids together with cholesterol, have received a lot of attention as they may resemble raft formation in real cells. Here we apply a simulation model to assess the molecular nature of these domains at the nanoscale, information that has thus far eluded experimental determination. We are able to show the spontaneous separation of a saturated phosphatidylcholine (PC)/unsaturated PC/cholesterol mixture into a liquid-ordered and a liquid-disordered phase with structural and dynamic properties closely matching experimental data. The near-atomic resolution of the simulations reveals remarkable features of both domains and the boundary domain interface. Furthermore, we predict the existence of a small surface tension between the monolayer leaflets that drives registration of the domains. At the level of molecular detail, raft-like lipid mixtures show a surprising face with possible implications for many cell membrane processes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
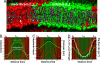
Formation of Lo domains in ternary lipid mixtures. (A) Color coding of the lipid components. Green is used for the saturated lipids, and red is used for the polyunsaturated lipids. Cholesterol is depicted in gray with a white hydroxyl group. (B) Time-resolved phase segregation of a planar membrane viewed from above, starting from a randomized mixture (t = 0), ending with the Lo/Ld coexistence (t = 20 μs). (C) Phase segregation for the same lipid mixture in a small, 20-nm-diameter liposome. Initial (t = 0) and final (t = 4 μs, both top view and cut through the middle) configuration. (D and E) Multiple periodic images (2 × 2) of the phase-separated diC16-PC/diC18:2-PC/cholesterol systems show striped pattern formation in the 0.42:0.28:0.3 system (D) and circular domains in the 0.28:0.42:0.3 system (E). (Scale bar: 5 nm.)
Fig. 2.
Structural and dynamic properties of the 2 domains. (A) Side view of the planar diC16-PC/diC18:2-PC/cholesterol 0.42:0.28:0.3 system at the end of the simulation, revealing the molecular organization in both the Lo and Ld phases. The white arrow points to a cholesterol oriented in between the monolayer leaflets. (B–D) Various properties of the membrane along the direction perpendicular to the phase boundaries. The Lo phase is centered, flanked by 2 periodic halves of the Ld domain. A transition zone separating the 2 phases is tentatively indicated by dashed, black lines. Green, red, and gray are used to distinguish properties of diC16-PC, diC18:2-PC, and cholesterol. (B) Composition of the membrane expressed as a mole fraction of each of the 3 components. Thin lines represent the 2 monolayers separately; the thicker line represents the average. (C) Average tail order parameter for PC lipids (left axis) and membrane thickness (black curve, right axis). (D) Lipid lateral diffusion rate (left axis) and cholesterol flip-flop rate (black curve, right axis).
Fig. 3.
Lateral organization of cholesterol in the Lo phase. (A) Top view of the planar membrane illustrating the typical instantaneous cholesterol organization across the raft-like phase and the boundary zone. The green and red backgrounds represent the positions of the saturated and unsaturated lipids. (B) Cholesterol–cholesterol radial distribution function with a solvent-separated peak at a distance of 1 nm (arrow). Results obtained from the current study are displayed by the solid line; results based on all-atom simulations (13) are shown by the dashed line for comparison. (Inset) Frequency distribution of cholesterol cluster sizes obtained from the simulation (solid bars) compared to a random distribution (open bars).
Fig. 4.
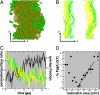
Driving forces for domain formation. (A) Image showing the overlap of the 2 raft domains at the end of the simulation (t = 20 μs). Only the CG beads corresponding to the phosphate group (PCs) or hydroxyl group (cholesterol) are shown as green solid spheres for the lower monolayer and transparent yellow spheres for the upper monolayer. The direction across the domains is indicated by x, and the direction along the domains is indicated by y. (B) Overlaying instantaneous configurations of the domain interfaces in the upper (yellow) and lower (green) monolayer leaflets during the last 4 μs of the simulation. Note the difference in fluctuations for the 2 innermost interfaces, which oppose the Lo phase, vs. the outermost interfaces opposing the Ld domain. (C) Minimization of the perimeter of the domain interface for each of the 2 monolayers (yellow and green curves, left axis) and the increase in registration between the domains formed in both monolayer leaflets, expressed as the surface overlap fraction (black curve, right axis). (D) Logarithmic probability of the area mismatch vs. area mismatch. The solid line denotes a linear fit of the data in the high mismatch regime, from which the effective surface tension between the monolayer leaflets is estimated.
Similar articles
- Regulating the size and stabilization of lipid raft-like domains and using calcium ions as their probe.
Szekely O, Schilt Y, Steiner A, Raviv U. Szekely O, et al. Langmuir. 2011 Dec 20;27(24):14767-75. doi: 10.1021/la203074q. Epub 2011 Nov 23. Langmuir. 2011. PMID: 22066979 - Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function.
Megha, London E. Megha, et al. J Biol Chem. 2004 Mar 12;279(11):9997-10004. doi: 10.1074/jbc.M309992200. Epub 2003 Dec 29. J Biol Chem. 2004. PMID: 14699154 - Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications.
Barenholz Y. Barenholz Y. Subcell Biochem. 2004;37:167-215. doi: 10.1007/978-1-4757-5806-1_5. Subcell Biochem. 2004. PMID: 15376621 Review. - Dynamics of raft molecules in the cell and artificial membranes: approaches by pulse EPR spin labeling and single molecule optical microscopy.
Subczynski WK, Kusumi A. Subczynski WK, et al. Biochim Biophys Acta. 2003 Mar 10;1610(2):231-43. doi: 10.1016/s0005-2736(03)00021-x. Biochim Biophys Acta. 2003. PMID: 12648777 Review.
Cited by
- Lysolipids regulate raft size distribution.
Krasnobaev VD, Galimzyanov TR, Akimov SA, Batishchev OV. Krasnobaev VD, et al. Front Mol Biosci. 2022 Oct 5;9:1021321. doi: 10.3389/fmolb.2022.1021321. eCollection 2022. Front Mol Biosci. 2022. PMID: 36275621 Free PMC article. - Structure and Dynamics of GPCRs in Lipid Membranes: Physical Principles and Experimental Approaches.
Jones AJY, Gabriel F, Tandale A, Nietlispach D. Jones AJY, et al. Molecules. 2020 Oct 15;25(20):4729. doi: 10.3390/molecules25204729. Molecules. 2020. PMID: 33076366 Free PMC article. Review. - Alteration of interleaflet coupling due to compounds displaying rapid translocation in lipid membranes.
Reigada R. Reigada R. Sci Rep. 2016 Sep 6;6:32934. doi: 10.1038/srep32934. Sci Rep. 2016. PMID: 27596355 Free PMC article. - Modeling the effects of lipid peroxidation during ferroptosis on membrane properties.
Agmon E, Solon J, Bassereau P, Stockwell BR. Agmon E, et al. Sci Rep. 2018 Mar 26;8(1):5155. doi: 10.1038/s41598-018-23408-0. Sci Rep. 2018. PMID: 29581451 Free PMC article. - Membrane texture induced by specific protein binding and receptor clustering: active roles for lipids in cellular function.
Watkins EB, Miller CE, Majewski J, Kuhl TL. Watkins EB, et al. Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):6975-80. doi: 10.1073/pnas.1014579108. Epub 2011 Apr 7. Proc Natl Acad Sci U S A. 2011. PMID: 21474780 Free PMC article.
References
- Jacobson K, Mouritsen OG, Anderson RGW. Lipid rafts: At a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. - PubMed
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. - PubMed
- Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. - PubMed
- Kahya N, Scherfeld D, Bacia K, Poolman B, Schwille P. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J Biol Chem. 2003;278:28109–28115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources