Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction - PubMed (original) (raw)
Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction
Michael Mederos y Schnitzler et al. EMBO J. 2008.
Abstract
Despite the central physiological function of the myogenic response, the underlying signalling pathways and the identity of mechanosensors in vascular smooth muscle (VSM) are still elusive. In contrast to present thinking, we show that membrane stretch does not primarily gate mechanosensitive transient receptor potential (TRP) ion channels, but leads to agonist-independent activation of G(q/11)-coupled receptors, which subsequently signal to TRPC channels in a G protein- and phospholipase C-dependent manner. Mechanically activated receptors adopt an active conformation, allowing for productive G protein coupling and recruitment of beta-arrestin. Agonist-independent receptor activation by mechanical stimuli is blocked by specific antagonists and inverse agonists. Increasing the AT(1) angiotensin II receptor density in mechanically unresponsive rat aortic A7r5 cells resulted in mechanosensitivity. Myogenic tone of cerebral and renal arteries is profoundly diminished by the inverse angiotensin II AT(1) receptor agonist losartan independently of angiotensin II (AII) secretion. This inhibitory effect is enhanced in blood vessels of mice deficient in the regulator of G-protein signalling-2. These findings suggest that G(q/11)-coupled receptors function as sensors of membrane stretch in VSM cells.
Figures
Figure 1
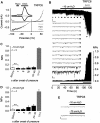
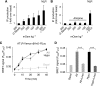
TRPC6 per se is not mechanosensitive. (A) Whole-cell recordings from HEK293 cells expressing TRPC6; application of hypotonic stimulus (250 mOsm kg−1), ‘Hypo', and of 100 μM OAG are indicated. Current time courses at ±60 mV, zero current levels (stippled lines), time scale bar (50 s) and current scale bar (200 pA). (B–E) Single-channel recordings in inside-out patches with negative pipette pressure and subsequent SAG bath application. (B) Original current trace, scale bar (2 pA, 10 s) (top); 13 consecutive traces on an expanded time-scale at the indicated time points, scale bar (5 pA, 20 ms) (middle); the respective graph of consecutive open probabilities (NPo) in 1-s steps (bottom) is displayed. (C, D) Analysis of mean NPo values. (E) Representative expanded current traces around the time point of pressure application, scale bar (5 pA, 10 ms).
Figure 2
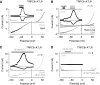
Indirect TRPC6 activation by membrane stretch through AT1R. Whole-cell recordings from transiently AT1R (A, B) or AT1R-Venus (C, D) and TRPC6 co-expressing HEK293 cells. (A–D) IV relationships before ‘Basal' and during hypotonicity (A, B) and direct membrane stretch (C, D) with and without 1 μM losartan, ‘Losa', are displayed; current time courses at ±60 mV, zero current levels (stippled lines), time scale bars: 50 s (A, B), 100 s (C, D) and current scale bars: 500 pA (A, B), 2 nA (C, D).
Figure 3
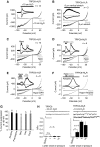
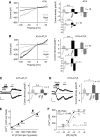
Inverse agonists and antagonists prevent mechanical activation of GPCRs. Whole-cell measurements of H1R (A–C, E) or M5R 100 s (D, F) with stimulation by positive pipette pressure (A), with vertical stretch (B) or with hypotonicity (C–F) in the presence or absence of 100 μM diphenhydramine, ‘DPH' (E) or 1 μM atropine (F). Agonist stimulation was performed with 100 μM histamine, ‘His' or with 100 μM carbachol ‘CCh'. Direct TRPC6 stimulation was performed with 100 μM OAG. Current time courses at ±60 mV, zero current levels (stippled lines), scale bars: 500 pA (A), 200 pA (B–E) or 1 nA (F) and 50 s (A, C–F) or 100 s (B). (G) Comparison of TRPC6 current suppression by atropine (1 μM), pirenzepine (1 μM), diphenhydramine (100 μM), losartan (1 μM) and candesartan, ‘Cand', (100 nM) with hypotonic stimulation of HEK293 cells co-expressing TRPC6 and the respective receptors. (H) Analysis of NPo values in cell-attached patches. (H) (top) Representative current traces before, during the first (left and right) and the fifths (right) second after application of −10 cm H2O pipette pressure. The closed state is indicated with ‘c' (right), scale bars (5 pA, 20 ms).
Figure 4
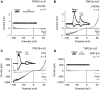
Osmotically induced membrane stretch activates phospholipase C and G proteins. (A–D) Whole-cell recordings from HEK293 cells co-expressing TRPC6 and H1R. Recording with 10 μM U73122 (A) or with 10 μM U73343 (B) in the pipette solution. Recordings of cells pretreated with 100 ng ml−1 PTX for 16 h (C) or with 2 mM GDP-β-S in the pipette solution (D). IV relationships before ‘Basal', during hypotonic stimulation, ‘Hypo' and receptor stimulation with 100 μM histamine, ‘His' are shown. (A–D) Insets show current time courses at ±60 mV, hypotonic and histamine stimulations, zero current levels (stippled lines); scale bars: 500 pA, 50 s.
Figure 5
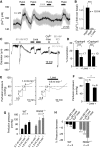
Active receptor conformations induced by membrane stretch. (A, B) Inositol phosphate (IP) accumulation as measured in one out of 3 representative assays with M5R expressing COS-7 cells. Agonist stimulation with 100 μM carbachol, ‘CCh', is also displayed. (C, D) BRET1 assay with COS-7 cells co-expressing AT1R-Venus and βArr2-_R_luc. (C) Representative time courses of BRET signals determined as quadruplets. (D) Summary of three independent BRET assays. After 30 min, BRET signals elicited by stimulation with an isotonic solution, ‘Basal', 100 nM AII, with a hypotonic solution, ‘Hypo' (273 mOsm kg−1), and under 1 μM losartan, ‘Losa'.
Figure 6
Expression of AT1R leads to mechanosensitivity of aortic SMCs. Whole-cell recordings from non-transfected (A) and A7r5 cells over-expressing AT1R (B–D). (A, B) IV relationships before ‘Basal', during hypotonic stimulation, ‘Hypo', and receptor stimulation with 1 μM vasopressin ‘VP' (left) and current density analyses at ±60 mV (right) are displayed. (C, D) Current time courses at ±60 mV with zero current levels (stippled lines); time scale bars 60 s and 100 pA (left) and current densities in the presence of neutralizing antibody, ‘nAb' (right) are displayed. (E) [Ca2+]i increases by hypotonic stimulation in HEK293 cells transfected with different amounts of AT1R-Venus cDNAs. Venus fluorescence was correlated with receptor densities. Numbers indicate the number of summarized cells and of independent transfections. (F) Concentration–response curves to AII of fura-2-loaded HEK293 cells stably expressing AT1R-Venus in isotonic and in hypotonic (273 mOsm kg−1) solutions.
Figure 7
Physiological relevance of agonist-independent activation of AT1R by membrane stretch. (A) Representative [Ca2+]i recordings in fura-2-loaded renal VSMCs. Application of hypotonic stimuli, ‘Hypo', and losartan, ‘Losa', are indicated. (B) Summary of maximal [Ca2+]i increases in renal VSMCs. (C) Representative trace of a diameter recording from an isolated rat cerebral artery continuously superfused with oxygenated PSS. Application of 60 mmHg intravascular pressure, applications of 60 mM high K+ solution, application of nominally Ca2+-free solution and applications of 1 μM losartan are represented. (D) Summary of measured cerebral arteries showing the reduction of vessel constriction with losartan in the presence or absence of 1 μM captopril. (E) Representative traces of perfusion pressure recordings from isolated kidneys of mice in the absence and presence of losartan. (F) Summary of perfusion pressures. (G) Summary of perfusion pressures at indicated flow rates measured in perfused isolated kidneys from wild-type, ‘WT', and RGS2−/− mice. (H) Summary of flow pressure decrease after 5 min measured at a flow rate of 1.9 ml min−1 illustrating the pressure decrease with losartan compared with a Ca2+-free bath solution. Captopril had no effect on perfusion pressure.
Comment in
- TRPCs, GPCRs and the Bayliss effect.
Voets T, Nilius B. Voets T, et al. EMBO J. 2009 Jan 7;28(1):4-5. doi: 10.1038/emboj.2008.261. EMBO J. 2009. PMID: 19129760 Free PMC article. No abstract available.
Similar articles
- Mechanosensitive Gq/11 Protein-Coupled Receptors Mediate Myogenic Vasoconstriction.
Mederos Y Schnitzler M, Storch U, Gudermann T. Mederos Y Schnitzler M, et al. Microcirculation. 2016 Nov;23(8):621-625. doi: 10.1111/micc.12293. Microcirculation. 2016. PMID: 27344060 Review. - Cysteinyl leukotriene 1 receptors as novel mechanosensors mediating myogenic tone together with angiotensin II type 1 receptors-brief report.
Storch U, Blodow S, Gudermann T, Mederos Y Schnitzler M. Storch U, et al. Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):121-6. doi: 10.1161/ATVBAHA.114.304844. Epub 2014 Nov 13. Arterioscler Thromb Vasc Biol. 2015. PMID: 25395620 - Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries.
Schleifenbaum J, Kassmann M, Szijártó IA, Hercule HC, Tano JY, Weinert S, Heidenreich M, Pathan AR, Anistan YM, Alenina N, Rusch NJ, Bader M, Jentsch TJ, Gollasch M. Schleifenbaum J, et al. Circ Res. 2014 Jul 7;115(2):263-72. doi: 10.1161/CIRCRESAHA.115.302882. Epub 2014 May 16. Circ Res. 2014. PMID: 24838176 - Myogenic Vasoconstriction Requires Canonical Gq/11 Signaling of the Angiotensin II Type 1 Receptor.
Cui Y, Kassmann M, Nickel S, Zhang C, Alenina N, Anistan YM, Schleifenbaum J, Bader M, Welsh DG, Huang Y, Gollasch M. Cui Y, et al. J Am Heart Assoc. 2022 Feb 15;11(4):e022070. doi: 10.1161/JAHA.121.022070. Epub 2022 Feb 8. J Am Heart Assoc. 2022. PMID: 35132870 Free PMC article. - Endocannabinoids modulate Gq/11 protein-coupled receptor agonist-induced vasoconstriction via a negative feedback mechanism.
Karpińska O, Baranowska-Kuczko M, Kloza M, Kozłowska H. Karpińska O, et al. J Pharm Pharmacol. 2018 Feb;70(2):214-222. doi: 10.1111/jphp.12854. Epub 2017 Nov 17. J Pharm Pharmacol. 2018. PMID: 29148061 Review.
Cited by
- Elementary calcium signaling in arterial smooth muscle.
Fan G, Cui Y, Gollasch M, Kassmann M. Fan G, et al. Channels (Austin). 2019 Dec;13(1):505-519. doi: 10.1080/19336950.2019.1688910. Channels (Austin). 2019. PMID: 31797713 Free PMC article. Review. - Tributyltin and Vascular Dysfunction: The Role of Oxidative Stress.
Ronconi KS, Stefanon I, Ribeiro Junior RF. Ronconi KS, et al. Front Endocrinol (Lausanne). 2018 Jul 12;9:354. doi: 10.3389/fendo.2018.00354. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30050498 Free PMC article. Review. - Impaired Cerebral Autoregulation After Subarachnoid Hemorrhage: A Quantitative Assessment Using a Mouse Model.
Koide M, Ferris HR, Nelson MT, Wellman GC. Koide M, et al. Front Physiol. 2021 Jun 8;12:688468. doi: 10.3389/fphys.2021.688468. eCollection 2021. Front Physiol. 2021. PMID: 34168571 Free PMC article. - What Evolutionary Evidence Implies About the Identity of the Mechanoelectrical Couplers in Vascular Smooth Muscle Cells.
Drummond HA. Drummond HA. Physiology (Bethesda). 2021 Sep 1;36(5):292-306. doi: 10.1152/physiol.00008.2021. Physiology (Bethesda). 2021. PMID: 34431420 Free PMC article. Review. - Angiotensin II Type 1 Receptor Mechanoactivation Involves RGS5 (Regulator of G Protein Signaling 5) in Skeletal Muscle Arteries: Impaired Trafficking of RGS5 in Hypertension.
Hong K, Li M, Nourian Z, Meininger GA, Hill MA. Hong K, et al. Hypertension. 2017 Dec;70(6):1264-1272. doi: 10.1161/HYPERTENSIONAHA.117.09757. Epub 2017 Oct 23. Hypertension. 2017. PMID: 29061726 Free PMC article.
References
- Ben-Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H (2006) Movement of ‘gating charge' is coupled to ligand binding in a G-protein-coupled receptor. Nature 444: 106–109 - PubMed
- Charest PG, Bouvier M (2003) Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances beta-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J Biol Chem 278: 41541–41551 - PubMed
- Clapham DE (2003) TRP channels as cellular sensors. Nature 426: 517–524 - PubMed
- Davis MJ, Hill MA (1999) Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials