A miRNA signature of prion induced neurodegeneration - PubMed (original) (raw)
A miRNA signature of prion induced neurodegeneration
Reuben Saba et al. PLoS One. 2008.
Abstract
MicroRNAs (miRNAs) are small, non-coding RNA molecules which are emerging as key regulators of numerous cellular processes. Compelling evidence links miRNAs to the control of neuronal development and differentiation, however, little is known about their role in neurodegeneration. We used microarrays and RT-PCR to profile miRNA expression changes in the brains of mice infected with mouse-adapted scrapie. We determined 15 miRNAs were de-regulated during the disease processes; miR-342-3p, miR-320, let-7b, miR-328, miR-128, miR-139-5p and miR-146a were over 2.5 fold up-regulated and miR-338-3p and miR-337-3p over 2.5 fold down-regulated. Only one of these miRNAs, miR-128, has previously been shown to be de-regulated in neurodegenerative disease. De-regulation of a unique subset of miRNAs suggests a conserved, disease-specific pattern of differentially expressed miRNAs is associated with prion-induced neurodegeneration. Computational analysis predicted numerous potential gene targets of these miRNAs, including 119 genes previously determined to be also de-regulated in mouse scrapie. We used a co-ordinated approach to integrate miRNA and mRNA profiling, bioinformatic predictions and biochemical validation to determine miRNA regulated processes and genes potentially involved in disease progression. In particular, a correlation between miRNA expression and putative gene targets involved in intracellular protein-degradation pathways and signaling pathways related to cell death, synapse function and neurogenesis was identified.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Classification of prion disease-specific miRNA target genes into, A, functional groups and B, canonical pathways.
Figure 2. Functional relationships of miRNA target genes involved in the ubiquitin-proteasome pathway and unfolded-protein response and cell death responses.
This diagram shows the direct (solid lines) and indirect (dashed lines) interactions reported for these putative target genes (grey shading) of miRNAs de-regulated in scrapie infection in mice. Biological network analysis was performed using Ingenuity Pathway Analysis (IPA).
Figure 3. A network of 29 miRNA target genes that have a high probability of interacting within neuronal synapses.
Figure 4. MiRNA target gene validation of EGR1 using a luciferase reporter gene assay.
A, Schematic representation of luciferase constructs used for reporter assays. Specifically, EGR1 3′UTR construct in pMIR-REPORT for transfection into HeLa cells. B, Dose dependent reduction in the expression of luciferase activity when HeLa cells are co-transfected with miR-203 and C, co-transfection with miR-191.
Figure 5. Comparison of the GO assignments for putative target genes of differentially expressed miRNAs with genes identified to be differentially regulated in prion diseases.
A, De-regulated target genes that show a correlation with de-regulated prion related genes. B, Functionally-related genes that are strongly de-regulated in mouse scrapie but have little or no representation among putative target genes of miRNAs that exhibit differential expression in prion disease (solid bars, genes de-regulated in mouse scrapie, hatched bars, putative targets of de-regulated miRNAs).
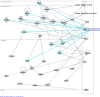
Figure 6. Functional relationships of de-regulated immune response-related genes in the brains of mice infected with scrapie and the putative transcription regulator genes that are targets of miRNAs similarly de-regulated.
This biological network illustrates the potential for indirect regulation of immune-response related genes by miRNAs via modulation of transcriptional regulators. Nodes colored in red denote up-regulated genes in mouse scrapie whereas green denotes down-regulated genes. Nodes with an orange border denote the putative targets of miRNAs deregulated in mouse scrapie.
References
- Booth S, Bowman C, Baumgartner R, Sorensen G, Robertson C, et al. Identification of central nervous system genes involved in the host response to the scrapie agent during preclinical and clinical infection. J Gen Virol. 2004;85(Pt 11):3459–3471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases