Two protonation switches control rhodopsin activation in membranes - PubMed (original) (raw)
Two protonation switches control rhodopsin activation in membranes
Mohana Mahalingam et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Activation of the G protein-coupled receptor (GPCR) rhodopsin is initiated by light-induced isomerization of the retinal ligand, which triggers 2 protonation switches in the conformational transition to the active receptor state Meta II. The first switch involves disruption of an interhelical salt bridge by internal proton transfer from the retinal protonated Schiff base (PSB) to its counterion, Glu-113, in the transmembrane domain. The second switch consists of uptake of a proton from the solvent by Glu-134 of the conserved E(D)RY motif at the cytoplasmic terminus of helix 3, leading to pH-dependent receptor activation. By using a combination of UV-visible and FTIR spectroscopy, we study the activation mechanism of rhodopsin in different membrane environments and show that these 2 protonation switches become partially uncoupled at physiological temperature. This partial uncoupling leads to approximately 50% population of an entropy-stabilized Meta II state in which the interhelical PSB salt bridge is broken and activating helix movements have taken place but in which Glu-134 remains unprotonated. This partial activation is converted to full activation only by coupling to the pH-dependent protonation of Glu-134 from the solvent, which stabilizes the active receptor conformation by lowering its enthalpy. In a membrane environment, protonation of Glu-134 is therefore a thermodynamic rather than a structural prerequisite for activating helix movements. In light of the conservation of the E(D)RY motif in rhodopsin-like GPCRs, protonation of this carboxylate also may serve a similar function in signal transduction of other members of this receptor family.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
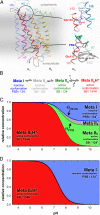
Fig. 1.
Thermodynamic and structural model for rhodopsin activation in a membrane environment. (A) The structure of the dark state of rhodopsin [based on Protein Data Bank ID code 1GZM (25)] includes 2 protonation switches. The cytoplasmic network around Glu-134 in the ERY motif of H3 is involved in proton uptake from the solvent, and the interhelical salt bridge between the protonated retinal Schiff base (PSB) linked to H7 and Glu-113 on H3 in the transmembrane domain is disrupted by internal proton transfer during receptor activation. (B) Previous studies (14, 18, 19) have proposed a sequential reaction scheme of coupled equilibria of photoproduct states for rhodopsin activation in a detergent environment, involving PSB deprotonation in the transition to Meta IIa, activating helix movements in the transition to Meta IIb, and cytoplasmic proton uptake by Glu-134 in the transition to Meta IIbH+. (The terms inactive and active are used to describe the receptor conformations before and after helix movements, respectively, and do not necessarily specify activity toward G protein.) (C) This reaction scheme predicts a complex titration behavior of photoproducts in a membrane environment, with Meta I, Meta IIa, and Meta IIb forming an equilibrium at alkaline pH. This intrinsically pH-independent equilibrium is coupled to a pH-dependent equilibrium with Meta IIbH+ at lower pH, reflecting proton uptake by Glu-134 from the solvent. (D) Because Meta IIa and Meta IIb are populated only at higher temperature, this 4-state scheme reduces to the classical 2-state Meta I/Meta IIbH+ scheme at lower temperature.
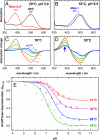
Fig. 2.
UV–visible spectroscopic characterization of the PSB salt bridge in the Meta I/Meta II equilibrium in native disk membranes. (A and B) At 20 °C and pH 5.0, photolysis converts the dark state of rhodopsin (black) to the Meta IIbH+ photoproduct state with deprotonated Schiff base (red), whereas, at 10 °C and pH 9.5, the Meta I photoproduct with protonated Schiff base (blue) is formed. (C) Difference spectra for the photoproduct minus dark state obtained at 10 °C reveal the pH dependence of the Meta I/Meta IIbH+ equilibrium, which is completely on the side of Meta IIbH+ at pH 5.0 and of Meta I at pH 9.0 and 10.0, as evident from the complete lack of absorption at 380 nm. (D) At 30 °C, the equilibrium is not fully shifted to Meta I at alkaline pH; instead a 380-nm Meta II photoproduct contribution is observed at very alkaline pH, which becomes pH-independent at pH 9.0 and above. (E) Schiff base deprotonation (θUV–vis) and, hence, the fraction of Meta II were determined from UV–visible difference spectra. At 10 °C the titration curve follows a regular Henderson–Hasselbalch function, whereas, at higher temperature, the alkaline endpoint θUV–visalk does not reach zero because of increasing population of the Meta IIa and Meta IIb states.
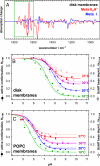
Fig. 3.
FTIR spectroscopic characterization of activating conformational changes in the Meta I/Meta II equilibrium in native disk membranes. (A) Meta I and Meta IIbH+ minus dark state FTIR difference spectra were obtained from rhodopsin in native disk membranes at 10 °C at pH 9.5 and 20 °C at pH 5.0, respectively, under identical conditions as in Fig. 2 A and B. By using the conformationally sensitive range marked in green, they served as basis spectra for inactive and active conformations, respectively, to determine the pH dependence of activating conformational changes. (B) The FTIR-based titration curves of receptor conformation, θFTIR (filled circles and solid lines), reveal a similar deviation from classical Henderson–Hasselbalch behavior as the UV–visible data, with a temperature-dependent increase of the alkaline endpoint θFTIRalk. The alkaline endpoints θUV–visalk of the UV–visible-based titration curves (open symbols, broken lines, reproduced from Fig. 2_E_) at 30 and 37 °C are slightly higher than θFTIRalk, revealing the additional contribution of inactive Meta IIa. (C) Rhodopsin reconstituted into synthetic POPC membranes shows the same general activation scheme but with shifted equilibria because of altered properties of the lipid bilayer. Both in native disk membranes and synthetic POPC membranes, proton uptake by Glu-134 and, hence, the transition to protonated Meta IIbH+ is necessary for full receptor activation.
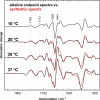
Fig. 4.
Meta IIb and Meta IIbH+ share a similar active state FTIR signature. FTIR difference spectra for the photoproduct minus dark state obtained for disk membranes at the alkaline endpoint reveal the increasing contribution of the active conformation of unprotonated Meta IIb to the Meta I/Meta IIa/Meta IIb photoproduct equilibrium at higher temperature. Synthetic Meta I/Meta IIbH+ minus dark state spectra (red) were calculated as a linear combination of the Meta I and Meta IIbH+ basis spectra shown in Fig. 3_A_ by using the endpoint values θUV–visalk (neglecting the small contribution of Meta IIa). These synthetic spectra show a remarkable agreement with the experimental endpoint spectra, indicating similar FTIR signatures of Meta IIb and Meta IIbH+.
Similar articles
- Coupling of protonation switches during rhodopsin activation.
Vogel R, Sakmar TP, Sheves M, Siebert F. Vogel R, et al. Photochem Photobiol. 2007 Mar-Apr;83(2):286-92. doi: 10.1562/2006-06-19-IR-937. Photochem Photobiol. 2007. PMID: 17576345 Review. - Modulating rhodopsin receptor activation by altering the pKa of the retinal Schiff base.
Vogel R, Siebert F, Yan EC, Sakmar TP, Hirshfeld A, Sheves M. Vogel R, et al. J Am Chem Soc. 2006 Aug 16;128(32):10503-12. doi: 10.1021/ja0627848. J Am Chem Soc. 2006. PMID: 16895417 - Rhodopsin activation switches in a native membrane environment.
Lüdeke S, Mahalingam M, Vogel R. Lüdeke S, et al. Photochem Photobiol. 2009 Mar-Apr;85(2):437-41. doi: 10.1111/j.1751-1097.2008.00490.x. Photochem Photobiol. 2009. PMID: 19267869 Review. - Functional role of the "ionic lock"--an interhelical hydrogen-bond network in family A heptahelical receptors.
Vogel R, Mahalingam M, Lüdeke S, Huber T, Siebert F, Sakmar TP. Vogel R, et al. J Mol Biol. 2008 Jul 18;380(4):648-55. doi: 10.1016/j.jmb.2008.05.022. Epub 2008 May 17. J Mol Biol. 2008. PMID: 18554610 - The role of Glu181 in the photoactivation of rhodopsin.
Lüdeke S, Beck M, Yan EC, Sakmar TP, Siebert F, Vogel R. Lüdeke S, et al. J Mol Biol. 2005 Oct 21;353(2):345-56. doi: 10.1016/j.jmb.2005.08.039. J Mol Biol. 2005. PMID: 16169009
Cited by
- The Activation Pathway of Human Rhodopsin in Comparison to Bovine Rhodopsin.
Kazmin R, Rose A, Szczepek M, Elgeti M, Ritter E, Piechnick R, Hofmann KP, Scheerer P, Hildebrand PW, Bartl FJ. Kazmin R, et al. J Biol Chem. 2015 Aug 14;290(33):20117-27. doi: 10.1074/jbc.M115.652172. Epub 2015 Jun 23. J Biol Chem. 2015. PMID: 26105054 Free PMC article. - Retinal Conformation Changes Rhodopsin's Dynamic Ensemble.
Leioatts N, Romo TD, Danial SA, Grossfield A. Leioatts N, et al. Biophys J. 2015 Aug 4;109(3):608-17. doi: 10.1016/j.bpj.2015.06.046. Biophys J. 2015. PMID: 26244742 Free PMC article. - Adaptation of pineal expressed teleost exo-rod opsin to non-image forming photoreception through enhanced Meta II decay.
Tarttelin EE, Fransen MP, Edwards PC, Hankins MW, Schertler GF, Vogel R, Lucas RJ, Bellingham J. Tarttelin EE, et al. Cell Mol Life Sci. 2011 Nov;68(22):3713-23. doi: 10.1007/s00018-011-0665-y. Epub 2011 Mar 17. Cell Mol Life Sci. 2011. PMID: 21416149 Free PMC article. - Crystallization scale preparation of a stable GPCR signaling complex between constitutively active rhodopsin and G-protein.
Maeda S, Sun D, Singhal A, Foggetta M, Schmid G, Standfuss J, Hennig M, Dawson RJ, Veprintsev DB, Schertler GF. Maeda S, et al. PLoS One. 2014 Jun 30;9(6):e98714. doi: 10.1371/journal.pone.0098714. eCollection 2014. PLoS One. 2014. PMID: 24979345 Free PMC article. - Insights into distinct signaling profiles of the µOR activated by diverse agonists.
Qu Q, Huang W, Aydin D, Paggi JM, Seven AB, Wang H, Chakraborty S, Che T, DiBerto JF, Robertson MJ, Inoue A, Suomivuori CM, Roth BL, Majumdar S, Dror RO, Kobilka BK, Skiniotis G. Qu Q, et al. Nat Chem Biol. 2023 Apr;19(4):423-430. doi: 10.1038/s41589-022-01208-y. Epub 2022 Nov 21. Nat Chem Biol. 2023. PMID: 36411392 Free PMC article.
References
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993;268:4625–4636. - PubMed
- Menon ST, Han M, Sakmar TP. Rhodopsin: Structural basis of molecular physiology. Physiol Rev. 2001;81:1659–1688. - PubMed
- Lewis JW, Kliger DS. Absorption spectroscopy in studies of visual pigments: Spectral and kinetic characterization of intermediates. Methods Enzymol. 2000;315:164–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources