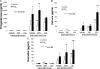Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis - PubMed (original) (raw)
Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis
Erkut Attar et al. J Clin Endocrinol Metab. 2009 Feb.
Abstract
Context: Products of at least five specific steroidogenic genes, including steroidogenic acute regulatory protein (StAR), which facilitates the entry of cytosolic cholesterol into the mitochondrion, side chain cleavage P450 enzyme, 3beta-hydroxysteroid-dehydrogenase-2, 17-hydroxylase/17-20-lyase, and aromatase, which catalyzes the final step, are necessary for the conversion of cholesterol to estrogen. Expression and biological activity of StAR and aromatase were previously demonstrated in endometriosis but not in normal endometrium. Prostaglandin E2 (PGE2) induces aromatase expression via the transcriptional factor steroidogenic factor-1 (SF1) in endometriosis, which is opposed by chicken-ovalbumin upstream-transcription factor (COUP-TF) and Wilms' tumor-1 (WT1) in endometrium.
Objective: The aim of the study was to demonstrate a complete steroidogenic pathway leading to estrogen biosynthesis in endometriotic cells and the transcriptional mechanisms that regulate basal and PGE2-stimulated estrogen production in endometriotic cells and endometrium.
Results: Compared with normal endometrial tissues, mRNA levels of StAR, side chain cleavage P450, 3beta-hydroxysteroid-dehydrogenase-2, 17-hydroxylase/17-20-lyase, aromatase, and SF1 were significantly higher in endometriotic tissues. PGE2 induced the expression of all steroidogenic genes; production of progesterone, estrone, and estradiol; and StAR promoter activity in endometriotic cells. Overexpression of SF1 induced, whereas COUP-TFII or WT1 suppressed, StAR promoter activity. PGE2 induced coordinate binding of SF1 to StAR and aromatase promoters but decreased COUP-TFII binding in endometriotic cells. COUP-TFII or WT1 binding to both promoters was significantly higher in endometrial compared with endometriotic cells.
Conclusion: Endometriotic cells contain the full complement of steroidogenic genes for de novo synthesis of estradiol from cholesterol, which is stimulated by PGE2 via enhanced binding of SF1 to promoters of StAR and aromatase genes in a synchronous fashion.
Figures
Figure 1
In vivo (tissue) mRNA levels of steroidogenic genes (real-time PCR). mRNA levels of the steroidogenic genes including the mitochondrial cholesterol transporter, StAR (STAR); the enzymes P450scc (CYP11A1), HSD3B2, P450c17 (CYP17A1), and P450arom (CYP19A1); and the transcription factor SF1 were found to be strikingly higher in tissues of endometriosis (n = 13) compared with endometrium (n = 13). On the other hand, mRNA levels of the SF1 homolog gene, LRH1, were higher in endometrial tissue. E-IUM, Endometrium; E-OSIS, endometriosis.
Figure 2
mRNA levels of steroidogenic genes in stromal cells isolated from normal eutopic endometrium vs. endometriosis incubated in the absence or presence of PGE2 (10−7
m
). PGE2 significantly stimulated mRNA levels of each steroidogenic gene except for HSD3B1 in endometriotic stromal cells (E-OSIS; n = 6). The mRNA levels of these genes in normal endometrium (E-IUM; n = 6) were very low regardless of the absence or presence of PGE2.
Figure 3
Hormone concentrations in media conditioned by stromal cells isolated from endometrium (n = 3) or endometriosis (n = 3) incubated in the absence or presence of PGE2 (10−7
m
), 22(R)-OH-cholesterol (22R; 10 μ
m
), or LDL (50 μg/ml). 22(R)-OH-cholesterol can diffuse into the mitochondria without a requirement for StAR. LDL was added in an attempt to increase the availability of cholesterol. PGE2 stimulated the levels progesterone (A), estrone (B), and estradiol (C) in media from endometriosis-derived stromal cells. The addition of 22R-OH-cholesterol or LDL did not modify this effect of PGE2 significantly, except for the enhancing effects of LDL on estrone levels.
Figure 4
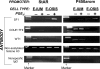
Time and dose-dependent induction of StAR mRNA and protein by PGE2 in endometriotic stromal cells. PGE2 stimulated StAR mRNA (A) and protein levels (B) in a time- and dose-dependent manner in endometriotic stromal cells. StAR protein in the last lane in the upper panel B was detected in endometriotic stromal cells treated for 48 h with dibutyryl cAMP. mRNA or protein levels of the house-keeping genes, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and actin, were used as loading controls.
Figure 5
Regulation of StAR promoter activity in endometriotic and endometrial cells. A, PGE2 stimulated the activity of the −885 bp StAR promoter fused to the luciferase (LUC) reporter gene in both endometriotic and endometrial cells, whereas it did not stimulate luciferase activity of the empty vector. *, P value for endometriotic cells; **, P value for endometrial cells. B, A vector overexpressing a transcription factor was cotransfected together with the −885 bp StAR-luciferase (LUC) into endometriotic or endometrial stromal cells. C/EBPβ, C/EBPα, SF1, or LRH1 induced StAR promoter activity in both cell types. On the other hand, WT1 or COUP-TFII inhibited StAR promoter activity. *, P value for endometriotic cells comparing an expression vector with the empty vector; **, P value for endometrial cells.
Figure 6
ChIP assay demonstrating coordinate binding activity of transcription factors to StAR and P450arom promoters. In endometriotic (E-OSIS) stromal cells, PGE2 induced binding of SF1 to both StAR and P450arom promoters, whereas very low SF1 binding was observed in endometrial (E-IUM) stromal cells. On the other hand, the inhibitory transcription factors COUP-TFII and WT1 were associated with StAR and P450arom promoters more avidly in endometrial cells compared with endometriotic cells. Note that PGE2 reduced binding of COUP-TFII or WT1 in endometriotic or endometrial cells. The association of the control protein, nonacetylated histone H3, to chromatin did not vary with cell type or treatment. No binding activity was observed after chromatin was immunoprecipitated with nonspecific IgG.
Similar articles
- Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis.
Utsunomiya H, Cheng YH, Lin Z, Reierstad S, Yin P, Attar E, Xue Q, Imir G, Thung S, Trukhacheva E, Suzuki T, Sasano H, Kim JJ, Yaegashi N, Bulun SE. Utsunomiya H, et al. Mol Endocrinol. 2008 Apr;22(4):904-14. doi: 10.1210/me.2006-0302. Epub 2007 Dec 28. Mol Endocrinol. 2008. PMID: 18165439 Free PMC article. - WT1 and DAX-1 inhibit aromatase P450 expression in human endometrial and endometriotic stromal cells.
Gurates B, Sebastian S, Yang S, Zhou J, Tamura M, Fang Z, Suzuki T, Sasano H, Bulun SE. Gurates B, et al. J Clin Endocrinol Metab. 2002 Sep;87(9):4369-77. doi: 10.1210/jc.2002-020522. J Clin Endocrinol Metab. 2002. PMID: 12213901 - Estrogen production in endometriosis and use of aromatase inhibitors to treat endometriosis.
Bulun SE, Zeitoun K, Takayama K, Noble L, Michael D, Simpson E, Johns A, Putman M, Sasano H. Bulun SE, et al. Endocr Relat Cancer. 1999 Jun;6(2):293-301. doi: 10.1677/erc.0.0060293. Endocr Relat Cancer. 1999. PMID: 10731122 Review. - Steroidogenic factor-1 and endometriosis.
Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, Tokunaga H, Trukhacheva E, Attar E, Gurates B, Milad MP, Confino E, Su E, Reierstad S, Xue Q. Bulun SE, et al. Mol Cell Endocrinol. 2009 Mar 5;300(1-2):104-8. doi: 10.1016/j.mce.2008.12.012. Epub 2008 Dec 25. Mol Cell Endocrinol. 2009. PMID: 19150483 Review.
Cited by
- Extracellular vesicle-associated VEGF-C promotes lymphangiogenesis and immune cells infiltration in endometriosis.
Li WN, Hsiao KY, Wang CA, Chang N, Hsu PL, Sun CH, Wu SR, Wu MH, Tsai SJ. Li WN, et al. Proc Natl Acad Sci U S A. 2020 Oct 13;117(41):25859-25868. doi: 10.1073/pnas.1920037117. Epub 2020 Oct 1. Proc Natl Acad Sci U S A. 2020. PMID: 33004630 Free PMC article. - Pathogenesis and pathophysiology of endometriosis.
Burney RO, Giudice LC. Burney RO, et al. Fertil Steril. 2012 Sep;98(3):511-9. doi: 10.1016/j.fertnstert.2012.06.029. Epub 2012 Jul 20. Fertil Steril. 2012. PMID: 22819144 Free PMC article. Review. - Epigenetic Factors in Eutopic Endometrium in Women with Endometriosis and Infertility.
Adamczyk M, Wender-Ozegowska E, Kedzia M. Adamczyk M, et al. Int J Mol Sci. 2022 Mar 30;23(7):3804. doi: 10.3390/ijms23073804. Int J Mol Sci. 2022. PMID: 35409163 Free PMC article. Review. - Deep Infiltrating Endometriosis and Adenomyosis: Implications on Pregnancy and Outcome.
Gruber TM, Ortlieb L, Henrich W, Mechsner S. Gruber TM, et al. J Clin Med. 2021 Dec 29;11(1):157. doi: 10.3390/jcm11010157. J Clin Med. 2021. PMID: 35011898 Free PMC article. Review. - The non-human primate model of endometriosis: research and implications for fecundity.
Braundmeier AG, Fazleabas AT. Braundmeier AG, et al. Mol Hum Reprod. 2009 Oct;15(10):577-86. doi: 10.1093/molehr/gap057. Epub 2009 Jul 24. Mol Hum Reprod. 2009. PMID: 19633013 Free PMC article. Review.
References
- Giudice LC, Kao LC 2004 Endometriosis. Lancet 364:1789–1799 - PubMed
- Ryan IP, Taylor RN 1997 Endometriosis and infertility: new concepts. Obstet Gynecol Surv 52:365–371 - PubMed
- Sampson JA 1927 Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14:422–469 - PubMed
- Tremblay Y, Ringler GE, Morel Y, Mohandas TK, Labrie F, Strauss 3rd JF, Miller WL 1989 Regulation of the gene for estrogenic 17-ketosteroid reductase lying on chromosome 17cen–q25. J Biol Chem 264:20458–20462 - PubMed
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J 2006 Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 248:94–103 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HD038691/HD/NICHD NIH HHS/United States
- R01 HD038691/HD/NICHD NIH HHS/United States
- HD40093/HD/NICHD NIH HHS/United States
- U54 HD040093/HD/NICHD NIH HHS/United States
- HD38691/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous