Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis - PubMed (original) (raw)
Review
Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis
Elena I Deryugina et al. Histochem Cell Biol. 2008 Dec.
Abstract
Since their introduction almost a century ago, chick embryo model systems involving the technique of chorioallantoic grafting have proved invaluable in the in vivo studies of tumor development and angiogenesis and tumor cell dissemination. The ability of the chick embryo's chorioallantoic membrane (CAM) to efficiently support the growth of inoculated xenogenic tumor cells greatly facilitates analysis of human tumor cell metastasis. During spontaneous metastasis, the highly vascularized CAM sustains rapid tumor formation within several days following cell grafting. The dense capillary network of the CAM also serves as a repository of aggressive tumor cells that escaped from the primary tumor and intravasated into the host vasculature. This spontaneous metastasis setting provides a unique experimental model to study in vivo the intravasation step of the metastatic cascade. During experimental metastasis when tumor cells are inoculated intravenously, the CAM capillary system serves as a place for initial arrest and then, for tumor cell extravasation and colonization. The tissue composition and accessibility of the CAM for experimental interventions makes chick embryo CAM systems attractive models to follow the fate and visualize microscopically the behavior of grafted tumor cells in both spontaneous and experimental metastasis settings.
Figures
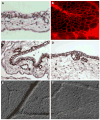
Fig. 1
Ectoderm capillary plexus of day 12 chick embryo. a H&E staining. b Immunofluorescent staining with Lens culinaris agglutinin (LCA). c, d Immunohistochemical staining with endothelium-specific lectin Sambuco negro agglutinin (SNA). d DIC microscopy of live, non-fixed whole mounts of the CAM at the ectoderm plexus level (e) and mesoderm level (f), ×100 (a, c) and ×200 (b, d–f)
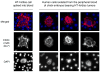
Fig. 2
Vasculotropism of HT-hi/diss cells escaped from primary CAM tumors. HT-lo/diss cells (A1) and HT-hi/diss cells (A2) were placed onto the CAM of day 10 incubation chick embryo. Paraffin sections of day 5 primary tumors were stained with anti-CD44 mAb 29-7 (brown) and counterstained with Mayer’s hematoxylin, ×200. HT-hi/diss cells in the vicinity of tumor/stroma border appear to be integrated into the wall of blood vessel (b), ×400. GFP-expressing HT-lo/diss cells (C1) and HT-hi/diss cells (C2) escaping CAM primary tumors were visualized in the embryos with the vasculature highlighted with red fluorescent-tagged LCA, ×400. d Quantitative analysis of HT-lo/diss and HT-hi/diss cells associated with blood vessels
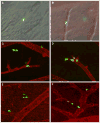
Fig. 3
Intravasated GFP-labeled HT-hi/diss cells visualized intravascularly (a–d) or appearing extravasating (e) and scattering (f) in the whole mount CAM preparations as visualized by DIC microscopy (a), DIC microscopy coupled with the highlighting of the CAM vasculature with the red-fluorescent LCA (b), and fluorescent microscopy (c–f), ×200
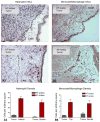
Fig. 4
Immunohistochemical staining of HT-hi/diss cells spontaneously metastasized to the liver of chick embryos bearing CAM tumors. Left HT-hi/diss cells localized intravascular, right extravascular micrometastatic foci, ×200
Fig. 5
Migration of HT-lo/diss (a) and HT-hi/diss (b) cells from the microtumors (left panels) developed in the CAM mesoderm. Intravital microscopy was performed in live embryos with an Olympus microscope equipped with a videocamera. Right panels depict enlarged portions of the frames originally taken with a ×20 objective
Fig. 6
Vasculotropism of HT-hi/diss cells escaped from microtumors developed on the CAM of the chick embryos grown ex ovo. a Shell-less chick embryo with HT-hi/diss microtumors (circles) developed for 5 days after grafting GFP-labeled cells within a drop of matrigel. b HT-hi/diss microtumor at larger magnification. c, d GFP-labeled HT-hi/diss cells visualized along CAM blood vessels by overlaying DIC and fluorescence digital images (c) or depicting overlaid immunofluorescent images only (c), ×200
Fig. 7
Influx of inflammatory cells to CAM tumors developed for 6 days from HT-lo/diss and HT-hi/diss cells. Heterophils (A1 and A2) were visualized with specific anti chicken MMP-9 antibody. Monocyte/macrophages (B1 and B2) were highlighted with anti-MMP-13 antibody, ×200. Density of inflammatory cells was determined in digital images and presented in the corresponding graphs (A3 and B3)
Fig. 8
Immunohistochemical staining (brown) of blood vessels in HT-lo/diss (a) and HT-hi/diss (b) tumors developed on the CAM of chick embryos. Paraffin sections were stained with Sambuco negro agglutinin specifically binding to chicken endothelial cells. Counterstaining was performed with Mayer’s hematoxylin, ×100. c Quantitation of lumina-containing blood vessels in SNA-stained sections of CAM tumors. d Quantitation of angiogenic blood vessels induced by HT-lo/diss and HT-hi/diss cells as determined in the collagen onplant assay (Deryugina and Quigley 2008)
Fig. 9
Detection of intravasated HT-hi/diss cells in circulation. Examples of human tumor cells (red) detected in the peripheral blood of the chick embryo. Peripheral blood (approximately 5 ml) was collected from allantoic vein 4 days after grafting HT-hi/diss cells (4 × 105) on the CAM of 10-day-old chick embryos. Peripheral blood was separated on discontinuous gradient of Histopaque. The cells from the band containing white blood cells and putative tumor cells were distributed on the slides, immunostained with human-specific mAb 29-7 against CD44, and processed by a FAST cytometer. Blue cell nuclei stained with DAPI (most stained nuclei represent chicken nucleated erythrocytes), ×400
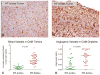
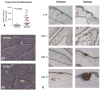
Fig. 10
Dissemination of HT-1080 intravasation variants in the experimental metastasis CAM model. Human HT-lo/diss (a) and HT-hi/diss (b) cells were inoculated i.v. into day 12 embryos and visualized within the CAM tissue 5 days later by immunostaining with mAb 29-7 specific to human CD44 (brown), ×200
Fig. 11
Analysis of experimental metastasis of congenic pair of colon carcinoma cell lines, SW480 and SW620, by Alu-qPCR (a), live microscopy of green fluorescent-tagged cells (b) and histological examination over time (d), ×100 (b), and ×40 (c)
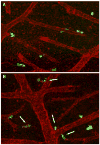
Fig. 12
Induction of HeLa–CDCP1 cell fragmentation by metastasis-blocking anti-CDCP1 mAb 41-2. HeLa cells transfected with CDCP1 were labeled with green fluorescent Tracker and inoculated i.v. into chick embryos along with control IgG (a) or anti-CDCP1 mAb 41-2 (b). Twelve hours after cell injections, the embryos were inoculated with red-fluorescent LCA to highlight the CAM vasculature. HeLa–CDCP1 cells were visualized in live, non-fixed whole mount preparations of the CAM. Arrows point to fragmented cells apparently undergoing apoptosis due to ligation of CDCP1 with mAb 41-2, ×200
Similar articles
- Quantitative Analysis of Human Cancer Cell Extravasation Using Intravital Imaging.
Willetts L, Bond D, Stoletov K, Lewis JD. Willetts L, et al. Methods Mol Biol. 2016;1458:27-37. doi: 10.1007/978-1-4939-3801-8_3. Methods Mol Biol. 2016. PMID: 27581012 - An Improved In Vivo Methodology to Visualise Tumour Induced Changes in Vasculature Using the Chick Chorionic Allantoic Membrane Assay.
Mangir N, Raza A, Haycock JW, Chapple C, Macneil S. Mangir N, et al. In Vivo. 2018 May-Jun;32(3):461-472. doi: 10.21873/invivo.11262. In Vivo. 2018. PMID: 29695547 Free PMC article. - Chick ex ovo culture and ex ovo CAM assay: how it really works.
Dohle DS, Pasa SD, Gustmann S, Laub M, Wissler JH, Jennissen HP, Dünker N. Dohle DS, et al. J Vis Exp. 2009 Nov 30;(33):1620. doi: 10.3791/1620. J Vis Exp. 2009. PMID: 19949373 Free PMC article. - Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis.
Ribatti D. Ribatti D. Int Rev Cell Mol Biol. 2008;270:181-224. doi: 10.1016/S1937-6448(08)01405-6. Int Rev Cell Mol Biol. 2008. PMID: 19081537 Review. - The CAM assay in the study of the metastatic process.
Ribatti D. Ribatti D. Exp Cell Res. 2021 Mar 15;400(2):112510. doi: 10.1016/j.yexcr.2021.112510. Epub 2021 Jan 29. Exp Cell Res. 2021. PMID: 33524363 Review.
Cited by
- Modern Photodynamic Glioblastoma Therapy Using Curcumin- or Parietin-Loaded Lipid Nanoparticles in a CAM Model Study.
Schulze J, Schöne L, Ayoub AM, Librizzi D, Amin MU, Engelhardt K, Yousefi BH, Bender L, Schaefer J, Preis E, Schulz-Siegmund M, Wölk C, Bakowsky U. Schulze J, et al. ACS Appl Bio Mater. 2023 Dec 18;6(12):5502-5514. doi: 10.1021/acsabm.3c00695. Epub 2023 Nov 28. ACS Appl Bio Mater. 2023. PMID: 38016693 Free PMC article. - Human Epidermal Growth Factor Receptor-3 Expression Is Regulated at Transcriptional Level in Breast Cancer Settings by Junctional Adhesion Molecule-A via a Pathway Involving Beta-Catenin and FOXA1.
Cruz RGB, Madden SF, Richards CE, Vellanki SH, Jahns H, Hudson L, Fay J, O'Farrell N, Sheehan K, Jirström K, Brennan K, Hopkins AM. Cruz RGB, et al. Cancers (Basel). 2021 Feb 19;13(4):871. doi: 10.3390/cancers13040871. Cancers (Basel). 2021. PMID: 33669586 Free PMC article. - Marine compounds inhibit growth of multiple myeloma in vitro and in vivo.
Steiner N, Ribatti D, Willenbacher W, Jöhrer K, Kern J, Marinaccio C, Aracil M, García-Fernández LF, Gastl G, Untergasser G, Gunsilius E. Steiner N, et al. Oncotarget. 2015 Apr 10;6(10):8200-9. doi: 10.18632/oncotarget.3362. Oncotarget. 2015. PMID: 25860931 Free PMC article. - Metallothionein-3 promotes cisplatin chemoresistance remodelling in neuroblastoma.
Rodrigo MAM, Michalkova H, Strmiska V, Casar B, Crespo P, de Los Rios V, Ignacio Casal J, Haddad Y, Guran R, Eckschlager T, Pokorna P, Heger Z, Adam V. Rodrigo MAM, et al. Sci Rep. 2021 Mar 9;11(1):5496. doi: 10.1038/s41598-021-84185-x. Sci Rep. 2021. PMID: 33750814 Free PMC article. - RAC1 induces nuclear alterations through the LINC complex to enhance melanoma invasiveness.
Colón-Bolea P, García-Gómez R, Shackleton S, Crespo P, Bustelo XR, Casar B. Colón-Bolea P, et al. Mol Biol Cell. 2020 Dec 1;31(25):2768-2778. doi: 10.1091/mbc.E20-02-0127. Epub 2020 Oct 7. Mol Biol Cell. 2020. PMID: 33026942 Free PMC article.
References
- Allan AL, Vantyghem SA, Tuck AB, Chambers AF, Chin-Yee IH, Keeney M. Detection and quantification of circulating tumor cells in mouse models of human breast cancer using immunomagnetic enrichment and multiparameter flow cytometry. Cytometry A. 2005;65:4–14. - PubMed
- Armstrong PB, Quigley JP, Sidebottom E. Transepithelial invasion and intramesenchymal infiltration of the chick embryo chorioallantois by tumor cell lines. Cancer Res. 1982;42:1826–1837. - PubMed
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. - PubMed
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. - PubMed
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev. 2006;6:24–37. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA055852/CA/NCI NIH HHS/United States
- R01 CA055852-16/CA/NCI NIH HHS/United States
- R01 CA105412/CA/NCI NIH HHS/United States
- R01 CA105412-05/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources