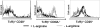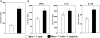Differential impact of L-arginine deprivation on the activation and effector functions of T cells and macrophages - PubMed (original) (raw)
Differential impact of L-arginine deprivation on the activation and effector functions of T cells and macrophages
B-S Choi et al. J Leukoc Biol. 2009 Feb.
Abstract
The metabolism of the amino acid L-arginine is emerging as a crucial mechanism for the regulation of immune responses. Here, we characterized the impact of L-arginine deprivation on T cell and macrophage (MPhi) effector functions: We show that whereas L-arginine is required unconditionally for T cell activation, MPhi can up-regulate activation markers and produce cytokines and chemokines in the absence of L-arginine. Furthermore, we show that L-arginine deprivation does not affect the capacity of activated MPhi to up-regulate L-arginine-metabolizing enzymes such as inducible NO synthase and arginase 1. Thus, our results show that to exert their effector functions, T cells and MPhi have different requirements for L-arginine.
Figures
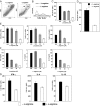
Fig. 1.
l
-arginine deprivation impairs T cells proliferation and cytokine production. Spleen cells or purified T cells were stimulated with anti-CD3 and anti-CD28 mAb in grading concentration of
l
-arginine (+
l
-arginine=400 μM; -
l
-arginine=0 μM). The capacity of T cells from total spleen (A–C) or purified T cells (D) to proliferate and the capacity of splenic T cells (E) and purified T cells (G) to produce cytokines were determined by flow cytometry. (F) The level of cytokine production was determined by ELISA. The error bars represent
sd
, and data show the results of one representative experiment out of four independent experiments.
Fig. 2.
l
-arginine deprivation impairs expression of activation markers by T cells. Spleen cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence (400 μM, black line) or absence (0 μM, gray line) of
l
-arginine, and the expression of CD28, CD62L, and CD25 on TcRβ+ cells was determined by flow cytometry. Data show the results of one representative experiment out of three independent experiments.
Fig. 3.
l
-arginine is essential for efficient effector functions following restimulation of activated T cells. Spleen cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence (400 μM) of
l
-arginine for 4 days, rested for 3 day, and restimulated for 24 h in the presence (400 μM) or absence (0 μM) of
l
-arginine. The capacity of T cells to proliferate (A) and produce cytokines (B) was determined by flow cytometry. The capacity of purified T cells from splenocytes to produce cytokines (C) was also determined. The error bars represent
sd
, and data show the results of one representative experiment out of three independent experiments.
Fig. 4.
l
-arginine depletion by arginase-expressing MΦ impairs T cells functions. Mature BMMΦs were stimulated with IL-4/IL-10 in the presence of 100 μM
l
-arginine. Two days later, MΦ were washed, and purified T cells (preactivated with anti-CD3 and anti-CD28 for 24 h) were added to the MΦ.
l
-arginine (400 μM) was added twice/day to some of the wells (+
l
-arginine). The capacity of T cells to proliferate (A) and produce cytokines (B) was determined by flow cytometry. The error bars represent
sd
, and data show the results of one representative experiment out of three independent experiments.
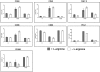
Fig. 5.
l
-arginine is not required for the expression of MΦ activation markers. Mature BMMΦ were differentiated in CAMΦ or AAMΦ or left unstimulated (nil) in the presence (400 μM, shaded bars) or absence (0 μM, open bars) of
l
-arginine, and the capacity to express cell-surface markers was analyzed by flow cytometry. The error bars represent
sd
, and data show the results of one representative experiment out of three independent experiments.
Fig. 6.
l
-arginine is not required for the expression of Ym1 and Fizz1, two hallmarks of AAMΦ and does not impair iNOS expression by CAMΦ. (A) Mature BMMΦ were differentiated in CAMΦ or AAMΦ or left unstimulated in the presence (400 μM) or absence (0 μM) of
l
-arginine, and the expression of Ym1, Fizz1, and iNOS was determined by Western blot. The same concentration of protein (14 μg) was loaded for each group. Data show the results of one representative experiment out of three independent experiments. (B) Mature BMMΦ were differentiated in CAMΦ or AAMΦ or left unstimulated in the presence (400 μM) or absence (0 μM) of
l
-arginine, and the production of NO was measured by the Griess reaction (B). The horizontal line represents the detection limit (5 μM). The error bars represent
sd
, and data show the results of one representative experiment out of five independent experiments. N.D., Not detected.
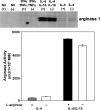
Fig. 7.
l
-arginine is not required for the expression and activity of arginase. Mature BMMΦ were activated with IFN-γ/TNF-α (CAMΦ), IL-4 (AAMΦ), or IL-4/IL-10 or left unstimulated in the presence (400 μM) or absence (0 μM) of
l
-arginine, and the expression of arginase was determined by Western blot (upper panel), and the activity of arginase was determined by enzymatic assay (lower panel). The same concentration of protein (14 μg) was loaded for each group. The error bars represent
sd
, and data show the results of one representative experiment out of five independent experiments.
Similar articles
- L-arginine deprivation impairs Leishmania major-specific T-cell responses.
Munder M, Choi BS, Rogers M, Kropf P. Munder M, et al. Eur J Immunol. 2009 Aug;39(8):2161-72. doi: 10.1002/eji.200839041. Eur J Immunol. 2009. PMID: 19637195 Free PMC article. - Arginine metabolism during macrophage autocrine activation and infection with mouse hepatitis virus 3.
Moreira C, Tsuhako MH, de Franco MT, Modolell M, Pereira CA. Moreira C, et al. Immunobiology. 2004;209(8):585-98. doi: 10.1016/j.imbio.2004.08.002. Immunobiology. 2004. PMID: 15638127 Free PMC article. - L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes.
Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. Rodriguez PC, et al. J Immunol. 2003 Aug 1;171(3):1232-9. doi: 10.4049/jimmunol.171.3.1232. J Immunol. 2003. PMID: 12874210 - Arginine availability, arginase, and the immune response.
Bansal V, Ochoa JB. Bansal V, et al. Curr Opin Clin Nutr Metab Care. 2003 Mar;6(2):223-8. doi: 10.1097/00075197-200303000-00012. Curr Opin Clin Nutr Metab Care. 2003. PMID: 12589193 Review. - Regulation of immune responses by L-arginine metabolism.
Bronte V, Zanovello P. Bronte V, et al. Nat Rev Immunol. 2005 Aug;5(8):641-54. doi: 10.1038/nri1668. Nat Rev Immunol. 2005. PMID: 16056256 Review.
Cited by
- Metabolomic profiling identifies distinct phenotypes for ASS1 positive and negative GBM.
Mörén L, Perryman R, Crook T, Langer JK, Oneill K, Syed N, Antti H. Mörén L, et al. BMC Cancer. 2018 Feb 8;18(1):167. doi: 10.1186/s12885-018-4040-3. BMC Cancer. 2018. PMID: 29422017 Free PMC article. - Polyamines: the pivotal amines in influencing the tumor microenvironment.
Holbert CE, Casero RA Jr, Stewart TM. Holbert CE, et al. Discov Oncol. 2024 May 18;15(1):173. doi: 10.1007/s12672-024-01034-9. Discov Oncol. 2024. PMID: 38761252 Free PMC article. Review. - Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms.
Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, Via LE, Barry CE 3rd, Klein E, Kirschner DE, Morris SM Jr, Lin PL, Flynn JL. Mattila JT, et al. J Immunol. 2013 Jul 15;191(2):773-84. doi: 10.4049/jimmunol.1300113. Epub 2013 Jun 7. J Immunol. 2013. PMID: 23749634 Free PMC article. - Similarity and diversity in macrophage activation by nematodes, trematodes, and cestodes.
Jenkins SJ, Allen JE. Jenkins SJ, et al. J Biomed Biotechnol. 2010;2010:262609. doi: 10.1155/2010/262609. Epub 2010 Jan 26. J Biomed Biotechnol. 2010. PMID: 20145705 Free PMC article. Review. - SSRP1/SLC3A2 Axis in Arginine Transport: A New Target for Overcoming Immune Evasion and Tumor Progression in Peripheral T-Cell Lymphoma.
Ren Y, Fan L, Wang L, Liu Y, Zhang J, Wang B, Chen R, Chen X, Zhuang L, Zhang Y, Sun H, Li J, Shi W, Jin H. Ren Y, et al. Adv Sci (Weinh). 2025 Jun;12(21):e2415698. doi: 10.1002/advs.202415698. Epub 2025 May 8. Adv Sci (Weinh). 2025. PMID: 40344476 Free PMC article.
References
- Popovic P J, Zeh H J, III, Ochoa J B. Arginine and immunity. J Nutr. 2007;137:1681S–1686S. - PubMed
- Morris S M., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137:1602S–1609S. - PubMed
- Munder M, Eichmann K, Moran J M, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. - PubMed
- Jost MM, Ninci E, Meder B, Kempf C, van Royen N, Hua J, Berger B, Hoefer I, Modolell M, Buschmann I. Divergent effects of GM-CSF and TGF-(1 on bone marrow-derived macrophage arginase-1 activity, MCP-1 expresssion and matrix-metalloproteinase-12: a potential role during arteriogenesis. FASEB J. 2003;17:2281–2283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials