Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging - PubMed (original) (raw)
Review
Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging
Cyrus M Ghajar et al. Histochem Cell Biol. 2008 Dec.
Abstract
The extracellular matrix (ECM), once thought to solely provide physical support to a tissue, is a key component of a cell's microenvironment responsible for directing cell fate and maintaining tissue specificity. It stands to reason, then, that changes in the ECM itself or in how signals from the ECM are presented to or interpreted by cells can disrupt tissue organization; the latter is a necessary step for malignant progression. In this review, we elaborate on this concept using the mammary gland as an example. We describe how the ECM directs mammary gland formation and function, and discuss how a cell's inability to interpret these signals -- whether as a result of genetic insults or physicochemical alterations in the ECM -- disorganizes the gland and promotes malignancy. By restoring context and forcing cells to properly interpret these native signals, aberrant behavior can be quelled and organization re-established. Traditional imaging approaches have been a key complement to the standard biochemical, molecular, and cell biology approaches used in these studies. Utilizing imaging modalities with enhanced spatial resolution in live tissues may uncover additional means by which the ECM regulates tissue structure, on different length scales, through its pericellular organization (short-scale) and by biasing morphogenic and morphostatic gradients (long-scale).
Figures
Fig. 1
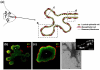
The intricate structure of the mammary gland can be recapitulated in 3D laminin-rich gels and is context-dependent. a The human mammary gland is composed, in part, of a bilayered epithelium consisting of luminal epithelial cells lining the duct and myoepithelial cells lining the basal surface. This tree-like structure is separated from the surrounding stroma by a basement membrane (BM) rich in laminins and collagens other than type I. Three-dimensional culture of murine mammary gland explants allows us to study primary branching or the formation of alveoli (alveologenesis), depending on the context (e.g., the type of ECM present). In a laminin-rich ECM (such as Matrigel), organoids undergo, b alveologenesis or c form acini depending on the soluble factors present. Keratin (K) staining reveals that proper polarity is achieved in this culture model: K8 (green), an epithelial marker, stains throughout these structures while K14 (red), indicative of myoepithelial cells in vivo, is confined to the basal surface (* denotes lumen). On the other hand, culturing organoids within a type I collagen matrix (d), upon stimulation by any of a number of growth factors, yields branched, primary duct-like structures with hollow lumens (inset). b and c were reproduced, with permission from Elsevier, from Fata et al. (2007). d was reproduced, with permission from The Company of Biologists Ltd., from Simian et al. (2001)
Fig. 2
Laminin _α_1 chain derived from a normal myoepithelium is necessary to confer polarity to luminal epithelial cells in a type I collagen gel. a While luminal epithelial cells (LEC) display proper organization and a basally-secreted basement membrane (BM) in a laminin-rich ECM (lrECM), b culture of LEC within a type I collagen (Coll I) gel results in disorganized structures which growth arrest but fail to deposit a BM. c Addition of myoepithelial cells (MEP) results in acini with proper polarity and restores formation of endogenous BM. d However, human breast cancer-derived MEP fail to confer polarity to LEC, as evidenced by a complete lack of lumen-containing structures within these cultures and disorganized staining of the apical marker sialomucin (green). This figure was reproduced with minor modifications from Gudjonsson et al. (2002) with permission from The Company of Biologists Ltd
Fig. 3
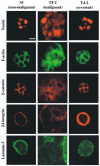
Treatment of malignant cells with reverting agents results in organized structures with proper polarity. Confocal microscopy of labeled nuclei, F-actin, _β_-catenin, _β_4 integrin, and laminin-5 reveals that while malignant cells form dense and disorganized clusters (middle column) marked by diffuse staining of F-actin, _β_-catenin, and integrin _β_4, and fail to deposit a basement membrane (BM), treating malignant cells with any of several reverting agents (_β_1 integrin targeting monoclonal antibody AIIB2 shown, right column) results in normalized clusters (compare to non-malignant cells, left column). These polarized clusters exhibit cortically organized F-actin, _β_-catenin concentrated at cell–cell junctions, basally localized _β_4 integrin, and basally secreted BM. This figure was reproduced, with permission from Elsevier, from Weaver et al. (2002)
Fig. 4
ECM composition and tissue architecture bias soluble factor gradients. To study the effects of ECM density on the passive diffusion of signaling molecules, the transport of fluorescein-tagged 10 kDa molecular weight dextran markers was monitored in microchannels containing a sparse (2.5 mg/ml fibrin) and b dense (10 mg/ml fibrin) tissues. After 25 min, it is clear that diffusion is greatly hindered in the dense matrix. c Effective diffusion coefficients (_D_eff) were extrapolated from these data for a range of molecular weight markers (presented as hydrodynamic radius, _R_H) to demonstrate the quantitative significance of this restriction († denotes P < 0.05 when comparing sparse to dense matrix conditions). These data are bounded by two well-known models of solute transport through fluid media (dotted and dashed lines). d The architecture of a tissue can also bias gradients of soluble factors. In this case, secretion of an inhibitory factor and its diffusion through a collagenous ECM was modeled computationally for mammary epithelial cell (MEC)-seeded tubules oriented perpendicular (left column) and parallel (right column) to each other. In both cases, inhibitor concentration was predicted to reach a maxim between the tubules (top row). Functionally, this predicted that MECs would branch in regions with reduced concentrations of the putative inhibitor. Heat maps (bottom row) generated from several images of tubules arranged in the described architectures illustrate that MECs do indeed branch in this fashion. Loss- and gain-of function studies demonstrated that the inhibitor in question was TGF-_β_1, and e fixing and staining these tissues for TGF-_β_1 demonstrates that the distribution of this factor matches that predicted by the computational model. Thus, knowing the distribution of an inhibitory gradient allows us to predict where branching will occur in a tissue. Developing techniques to image gradients within live tissues would allow the investigation of how the distribution of factors critical to maintaining homeostasis are disrupted by physicochemical changes within the matrix at the onset of malignancy. a–c of this figure were reproduced, with permission from The Biophysical Society, from Ghajar et al. (2008), while d and e were reproduced, with the permission from the American Association for the Advancement of Science, from Nelson et al. (2006)
Similar articles
- Integrated extracellular matrix signaling in mammary gland development and breast cancer progression.
Zhu J, Xiong G, Trinkle C, Xu R. Zhu J, et al. Histol Histopathol. 2014 Sep;29(9):1083-92. doi: 10.14670/HH-29.1083. Epub 2014 Mar 28. Histol Histopathol. 2014. PMID: 24682974 Free PMC article. Review. - Stem Cells and the Differentiation Hierarchy in Mammary Gland Development.
Fu NY, Nolan E, Lindeman GJ, Visvader JE. Fu NY, et al. Physiol Rev. 2020 Apr 1;100(2):489-523. doi: 10.1152/physrev.00040.2018. Epub 2019 Sep 20. Physiol Rev. 2020. PMID: 31539305 Review. - Mammary development and breast cancer: the role of stem cells.
Ercan C, van Diest PJ, Vooijs M. Ercan C, et al. Curr Mol Med. 2011 Jun;11(4):270-85. doi: 10.2174/156652411795678007. Curr Mol Med. 2011. PMID: 21506923 Free PMC article. Review. - Mechanisms by which the extracellular matrix and integrin signaling act to regulate the switch between tumor suppression and tumor promotion.
Keely PJ. Keely PJ. J Mammary Gland Biol Neoplasia. 2011 Sep;16(3):205-19. doi: 10.1007/s10911-011-9226-0. Epub 2011 Aug 7. J Mammary Gland Biol Neoplasia. 2011. PMID: 21822945 Free PMC article. Review. - Developmental biology: cell fate in the mammary gland.
Tong Q, Hotamisligil GS. Tong Q, et al. Nature. 2007 Feb 15;445(7129):724-6. doi: 10.1038/445724a. Nature. 2007. PMID: 17301782 No abstract available.
Cited by
- Identification of the new molecular subtypes related to inflammation in breast cancer.
Yu K, Xu C, Wang F, Wang H. Yu K, et al. Medicine (Baltimore). 2024 May 10;103(19):e38146. doi: 10.1097/MD.0000000000038146. Medicine (Baltimore). 2024. PMID: 38728446 Free PMC article. - A closer look into the cellular and molecular biology of myoepithelial cells across various exocrine glands.
Mauduit O, Delcroix V, Wong A, Ivanova A, Miles L, Lee HS, Makarenkova H. Mauduit O, et al. Ocul Surf. 2024 Jan;31:63-80. doi: 10.1016/j.jtos.2023.12.003. Epub 2023 Dec 21. Ocul Surf. 2024. PMID: 38141817 Free PMC article. Review. - Reconstruction of dynamic mammary mini gland in vitro for normal physiology and oncogenesis.
Yuan L, Xie S, Bai H, Liu X, Cai P, Lu J, Wang C, Lin Z, Li S, Guo Y, Cai S. Yuan L, et al. Nat Methods. 2023 Dec;20(12):2021-2033. doi: 10.1038/s41592-023-02039-y. Epub 2023 Nov 2. Nat Methods. 2023. PMID: 37919421 - FGFR2 Controls Growth, Adhesion and Migration of Nontumorigenic Human Mammary Epithelial Cells by Regulation of Integrin β1 Degradation.
Mieczkowski K, Popeda M, Lesniak D, Sadej R, Kitowska K. Mieczkowski K, et al. J Mammary Gland Biol Neoplasia. 2023 May 16;28(1):9. doi: 10.1007/s10911-023-09537-x. J Mammary Gland Biol Neoplasia. 2023. PMID: 37191822 Free PMC article. - The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis.
Dzobo K, Dandara C. Dzobo K, et al. Biomimetics (Basel). 2023 Apr 5;8(2):146. doi: 10.3390/biomimetics8020146. Biomimetics (Basel). 2023. PMID: 37092398 Free PMC article. Review.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. Garland Science; New York: 2002.
- Alford D, Baeckstrom D, Geyp M, Pitha P, Taylor-Papadimitriou J. Integrin-matrix interactions affect the form of the structures developing from human mammary epithelial cells in collagen or fibrin gels. J Cell Sci. 1998;111(Pt 4):521–532. - PubMed
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. - PubMed
- Ben-Ze’ev A, Farmer SR, Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979;17:319–325. - PubMed
Publication types
MeSH terms
Grants and funding
- U54 CA126552-05/CA/NCI NIH HHS/United States
- U54 CA112970-01/CA/NCI NIH HHS/United States
- U54 CA126552/CA/NCI NIH HHS/United States
- U54 CA143836-01/CA/NCI NIH HHS/United States
- U54 CA112970/CA/NCI NIH HHS/United States
- R01 CA057621-08/CA/NCI NIH HHS/United States
- R01 CA057621/CA/NCI NIH HHS/United States
- R01 CA064786-08/CA/NCI NIH HHS/United States
- U01 CA143233/CA/NCI NIH HHS/United States
- R01 CA064786/CA/NCI NIH HHS/United States
- U01 CA143233-01/CA/NCI NIH HHS/United States
- U54 CA143836/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical