Ubiquitin proteasome system stress underlies synergistic killing of ovarian cancer cells by bortezomib and a novel HDAC6 inhibitor - PubMed (original) (raw)
Ubiquitin proteasome system stress underlies synergistic killing of ovarian cancer cells by bortezomib and a novel HDAC6 inhibitor
Martina Bazzaro et al. Clin Cancer Res. 2008.
Abstract
Purpose: Elevated metabolic activity of ovarian cancer cells causes increased ubiquitin-proteasome-system (UPS) stress, resulting in their greater sensitivity to the toxic effects of proteasomal inhibition. The proteasomes and a potentially compensatory histone deacetylase 6 (HDAC6)-dependent lysosomal pathway mediate eukaryotic protein turnover. We hypothesized that up-regulation of the HDAC6-dependent lysosomal pathway occurs in response to UPS stress and proteasomal inhibition, and thus, ovarian cancer cell death can be triggered most effectively by coinhibition of both the proteasome- and HDAC6-dependent protein degradation pathways.
Experimental design: To address this hypothesis, we examined HDAC6 expression patterns in normal and cancerous ovarian tissues and used a novel HDAC6-specific inhibitor, NK84, to address HDAC6 function in ovarian cancer.
Results: Abnormally high levels of HDAC6 are expressed by ovarian cancer cells in situ and in culture relative to benign epithelium and immortalized ovarian surface epithelium, respectively. Specific HDAC6 inhibition acts in synergy with the proteasome inhibitor Bortezomib (PS-341) to cause selective apoptotic cell death of ovarian cancer cells at doses that do not cause significant toxicity when used individually. Levels of UPS stress regulate the sensitivity of ovarian cancer cells to proteasome/HDAC6 inhibition. Pharmacologic inhibition of HDAC6 also reduces ovarian cancer cell spreading and migration consistent with its known function in regulating microtubule polymerization via deacetylation of alpha-tubulin.
Conclusion: Our results suggest the elevation of both the proteasomal and alternate HDAC6-dependent proteolytic pathways in ovarian cancer and the potential of combined inhibition of proteasome and HDAC6 as a therapy for ovarian cancer.
Conflict of interest statement
Conflict of Interest: The authors declare none.
Figures
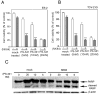
Figure 1. HDAC6 over-expression in ovarian carcinoma and cell lines
A, left panel,immunohistochemical staining of HDAC6 in ovarian tumors. Representative examples of intense HDAC6 staining of high-grade and low-grade ovarian serous carcinomas and weaker staining in serous adenofibroma and serous cystadenoma (imaging with X40 objective), right panel, staining intensity for each case was graded as 0 (no staining), 1 (weak staining), 2 (moderate staining) and 3 (intense staining), and the statistical significance of differences in staining intensities among indicated groups was calculated using the Mann-Whitney U test. Error bars indicate ± SD. B, Western blot analysis of HDAC6 in clinical specimens of serous cystadenoma (lanes 1–4) and serous carcinoma (lanes 5–10). Equal loading was verified by using an antibody directed against _β_-actin. C, Western blot analysis of HDAC6 immortalized ovarian surface epithelial cells (IOSE-29 and IOSE-397) and ovarian cancer cell lines (SKOV-3, ES-2, TOV-21G). Equal protein loading in each lane was verified by using an antibody directed against _β-_actin.
Figure 2. NK84 treatment reduces the viability ovarian cancer cells while sparing IOSEs
A, Ovarian cancer cell lines (SKOV-3, ES-2, TOV-21G) and immortalized ovarian surface epithelial cells (IOSE-29 and IOSE-397) were treated with the HDAC6 inhibitor NK84 (A,B) at the concentrations indicated. Cell viability was measured by XTT assay after culturing the cells for 24 hours (A) or 48 hours (B) in presence of NK84 inhibitor. Error bars indicate ± SD.
Figure 3. Simultaneous inhibition of HDAC6 and proteasome activity induces synergistic killing of ovarian cancer cells
Dose-dependent inhibition of the cell viability of ES-2 (A) and TOV-21G (B) in the absence (−) or in the presence (+) of 5μM NK84 HDAC6 inhibitor and PS-341 at the indicated concentration. Cell viability was measured after a 24 hours incubation by XTT assay and the percentage of viable cells is presented relative to mock-treated controls. ***P < 0.001. Error bars indicate ± SD. Lysate of ES-2 cell line treated with NK84 (5 μm) and/or PS-341 at the indicated concentrations was immunoblotted with an antibody recognizing the full-length and cleaved forms of PARP. Equal protein loading was verified by using an antibody directed against β-actin.
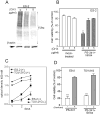
Figure 4. Synergistic activity of PS-341 and NK84 on ovarian cancer cells is dependent upon level of metabolic activity
A, Immunoblot analysis of the levels of poly-ubiquitinated proteins in cyclohexamide-exposed (24h) ES-2 ovarian cancer cells. Equal loading was verified by using an antibody directed against _β_-actin. B, ES-2 ovarian cancer cells pre-exposed to 0, 1 or 5 mg/ml CHX (24 hours) subsequently received treatment with of PS-341 (1nM) and NK84 (5 μM) or mock treatment. Cell viability was as evaluated by XTT assay after 24 hours of treatment. Percentage of viable cells is relative to mock-treated control cells is presented. ** P < 0.02. Error bars indicate ± SD. C, proliferation rate of confluent (+) or sub-confluent (−) ES-2 and TOV-21G ovarian cancer cell lines was measured by XTT assay. Each assay was performed in triplicate. Shown are bars ± SD of proliferative activity measured in terms of optical density at 450 nm on each given day. D, cell viability of ES-2 and TOV-21G ovarian cancer cell lines was evaluated by XTT assay in sub-confluent (−) versus confluent (+) cultures in the presence of PS-341 1nM and NK84 5 μM. Percentage of viable cells is relative to mock-treated control cells. ***P < 0.001. Error bars indicate ± SD.
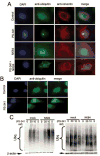
Figure 5. HDAC6 inhibition prevents aggresome formation in UPS stressed ovarian cancer cells
A, ES-2 ovarian cancer cells were incubated with 5nM PS-341, 10 μM NK84 or the combination of both for 24 hours before fixation and immuno-fluorescent staining of DNA (blue), ubiquitin (green) and vimentin (red) and imaging (X60 objective). B, IOSE-29 cells were incubated with 20nM PS-341 for 24 hours before fixation and immuno-fluorescent staining of DNA (blue), ubiquitin (green) and vimentin (red) and imaging (X60 objective). C, Immunoblot analysis of ubiquitinated protein in ES-2 cells (left panel) or IOSE-29 cells (right panel) 24h after treatment with or without 10 μM NK84 and the indicated concentrations of PS-341. Equal protein loading in each lane was verified by using an antibody directed against _β_-actin.
Figure 6. Pharmacologic inhibition of HDAC6 impedes cell motility and migration of ovarian cancer cells
A Plates of confluent SKOV-3 cells either mock or NK84 treated (10 μM) were examined by phase-contrast microscopy at the time of removal by scratching (0 hours) and 5 hours later. B, Analysis of the number of SKOV-3 cells spreading across the wound; results are means ± SD of three independent experiments. C, an equivalent number (2.5 × 104) of SKOV-3 or ES-3 cells mock-treated or treated with 10 μM of NK84 HDAC6 inhibitor were seeded in migration chambers and migrating cells were counted per each condition 8 hours thereafter. Three different fields of cells for each condition were counted at 40X. Each assay was performed in triplicate. Shown are means ± SD of migrating cells per microscopic field. D, representative example of migration assay conducted on mock or NK84-treated SKOV-3 cells.
Similar articles
- Ubiquitin-proteasome system stress sensitizes ovarian cancer to proteasome inhibitor-induced apoptosis.
Bazzaro M, Lee MK, Zoso A, Stirling WL, Santillan A, Shih IeM, Roden RB. Bazzaro M, et al. Cancer Res. 2006 Apr 1;66(7):3754-63. doi: 10.1158/0008-5472.CAN-05-2321. Cancer Res. 2006. PMID: 16585202 - Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma.
Hideshima T, Bradner JE, Wong J, Chauhan D, Richardson P, Schreiber SL, Anderson KC. Hideshima T, et al. Proc Natl Acad Sci U S A. 2005 Jun 14;102(24):8567-72. doi: 10.1073/pnas.0503221102. Epub 2005 Jun 3. Proc Natl Acad Sci U S A. 2005. PMID: 15937109 Free PMC article. - Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma.
Santo L, Hideshima T, Kung AL, Tseng JC, Tamang D, Yang M, Jarpe M, van Duzer JH, Mazitschek R, Ogier WC, Cirstea D, Rodig S, Eda H, Scullen T, Canavese M, Bradner J, Anderson KC, Jones SS, Raje N. Santo L, et al. Blood. 2012 Mar 15;119(11):2579-89. doi: 10.1182/blood-2011-10-387365. Epub 2012 Jan 19. Blood. 2012. PMID: 22262760 Free PMC article. - The ubiquitin-proteasome system (UPS) and the mechanism of action of bortezomib.
Cvek B, Dvorak Z. Cvek B, et al. Curr Pharm Des. 2011;17(15):1483-99. doi: 10.2174/138161211796197124. Curr Pharm Des. 2011. PMID: 21504411 Review. - Antiproliferative and proapoptotic effects of proteasome inhibitors and their combination with histone deacetylase inhibitors on leukemia cells.
Fuchs O, Provaznikova D, Marinov I, Kuzelova K, Spicka I. Fuchs O, et al. Cardiovasc Hematol Disord Drug Targets. 2009 Mar;9(1):62-77. doi: 10.2174/187152909787581372. Cardiovasc Hematol Disord Drug Targets. 2009. PMID: 19275578 Review.
Cited by
- Histone deacetylase 6 in cancer.
Li T, Zhang C, Hassan S, Liu X, Song F, Chen K, Zhang W, Yang J. Li T, et al. J Hematol Oncol. 2018 Sep 3;11(1):111. doi: 10.1186/s13045-018-0654-9. J Hematol Oncol. 2018. PMID: 30176876 Free PMC article. Review. - Glycosylation-Related Genes Predict the Prognosis and Immune Fraction of Ovarian Cancer Patients Based on Weighted Gene Coexpression Network Analysis (WGCNA) and Machine Learning.
Zhao C, Xiong K, Zhao F, Adam A, Li X. Zhao C, et al. Oxid Med Cell Longev. 2022 Mar 4;2022:3665617. doi: 10.1155/2022/3665617. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35281472 Free PMC article. - Advances in Targeting the Epidermal Growth Factor Receptor Pathway by Synthetic Products and Its Regulation by Epigenetic Modulators As a Therapy for Glioblastoma.
Nadeem Abbas M, Kausar S, Wang F, Zhao Y, Cui H. Nadeem Abbas M, et al. Cells. 2019 Apr 12;8(4):350. doi: 10.3390/cells8040350. Cells. 2019. PMID: 31013819 Free PMC article. Review. - Distinct methylation profile of mucinous ovarian carcinoma reveals susceptibility to proteasome inhibitors.
Liew PL, Huang RL, Weng YC, Fang CL, Hui-Ming Huang T, Lai HC. Liew PL, et al. Int J Cancer. 2018 Jul 15;143(2):355-367. doi: 10.1002/ijc.31324. Epub 2018 Mar 8. Int J Cancer. 2018. PMID: 29451304 Free PMC article. - Epigenetic heterogeneity promotes acquired resistance to BET bromodomain inhibition in ovarian cancer.
Sun Y, Zhang Z, Zhang K, Liu Y, Shen P, Cai M, Jia C, Wang W, Gu Z, Ma P, Lu H, Guan L, Di W, Zhuang G, Yin X. Sun Y, et al. Am J Cancer Res. 2021 Jun 15;11(6):3021-3038. eCollection 2021. Am J Cancer Res. 2021. PMID: 34249442 Free PMC article.
References
- Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Cell Death Differ. 2005;12:1178–90. - PubMed
- Aghajanian C. Clinical update: novel targets in gynecologic malignancies. Semin Oncol. 2004;31:22–6. discussion 33. - PubMed
- Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Proteasome inhibition in the treatment of cancer. Cell Cycle. 2005;4:290–6. - PubMed
- Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630–9. - PubMed
- Bazzaro M, Lee MK, Zoso A, et al. Ubiquitin-proteasome system stress sensitizes ovarian cancer to proteasome inhibitor-induced apoptosis. Cancer Res. 2006;66:3754–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA122581/CA/NCI NIH HHS/United States
- P50 CA098252/CA/NCI NIH HHS/United States
- R01 CA122581-03/CA/NCI NIH HHS/United States
- R01CA122581/CA/NCI NIH HHS/United States
- P50 CA098252-05/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical